5) The following are actual calculated molecular orbitals for the diatomic molecule OF- (note the minus sign indicates that your MO diagram should contain an extra electron). The solid blue (left) and red (right) spheres represent fluorine and oxygen respectively. The wireframe represents the molecular orbital (red and black are different phases). The coordinate system is provided. For each orbital: (i) label the molecular orbital as you would in an MO diagram given the rules we have learned in class for labeling MO diagrams (ii) identify the main atomic orbitals that it is constructed from and from which atom they originate (iii) draw a schematic picture of it (Just the basic orbital shapes of s or p atomic orbitals with the correct phase). An example is given below. Calculated M0: (i) Label: 10* (ii) F(2s) – 0(2s) F 0 (ii)
5) The following are actual calculated molecular orbitals for the diatomic molecule OF- (note the minus sign indicates that your MO diagram should contain an extra electron). The solid blue (left) and red (right) spheres represent fluorine and oxygen respectively. The wireframe represents the molecular orbital (red and black are different phases). The coordinate system is provided. For each orbital: (i) label the molecular orbital as you would in an MO diagram given the rules we have learned in class for labeling MO diagrams (ii) identify the main atomic orbitals that it is constructed from and from which atom they originate (iii) draw a schematic picture of it (Just the basic orbital shapes of s or p atomic orbitals with the correct phase). An example is given below. Calculated M0: (i) Label: 10* (ii) F(2s) – 0(2s) F 0 (ii)
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.101QE: The molecular orbital diagram of NO shown in Figure 10.47 also applies to OF. Draw the complete...
Related questions
Question
Help me please
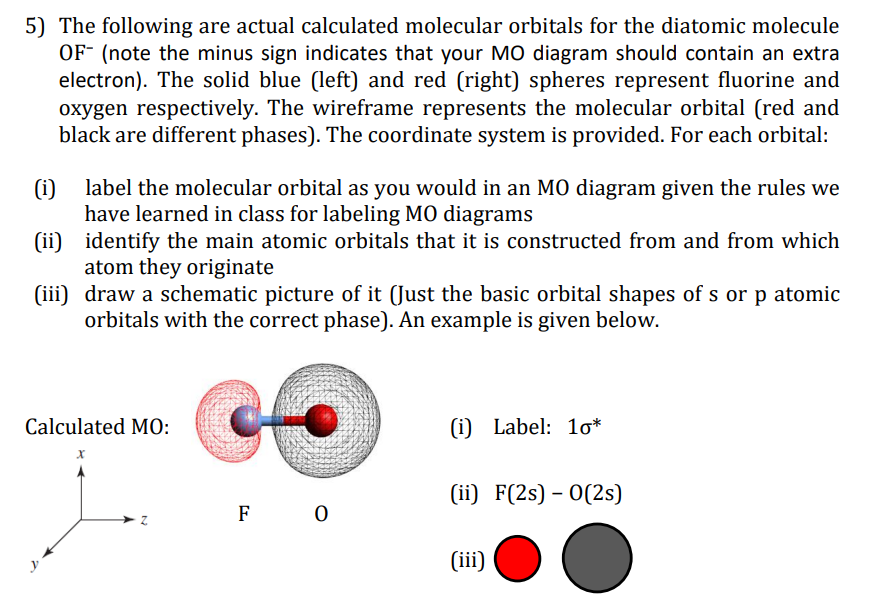
Transcribed Image Text:5) The following are actual calculated molecular orbitals for the diatomic molecule
OF- (note the minus sign indicates that your MO diagram should contain an extra
electron). The solid blue (left) and red (right) spheres represent fluorine and
oxygen respectively. The wireframe represents the molecular orbital (red and
black are different phases). The coordinate system is provided. For each orbital:
(i)
label the molecular orbital as you would in an MO diagram given the rules we
have learned in class for labeling MO diagrams
(ii) identify the main atomic orbitals that it is constructed from and from which
atom they originate
(iii) draw a schematic picture of it (Just the basic orbital shapes of s or p atomic
orbitals with the correct phase). An example is given below.
Calculated MO:
(i) Label: 1o*
(ii) F(2s) – O(2s)
F 0
y
(iii)
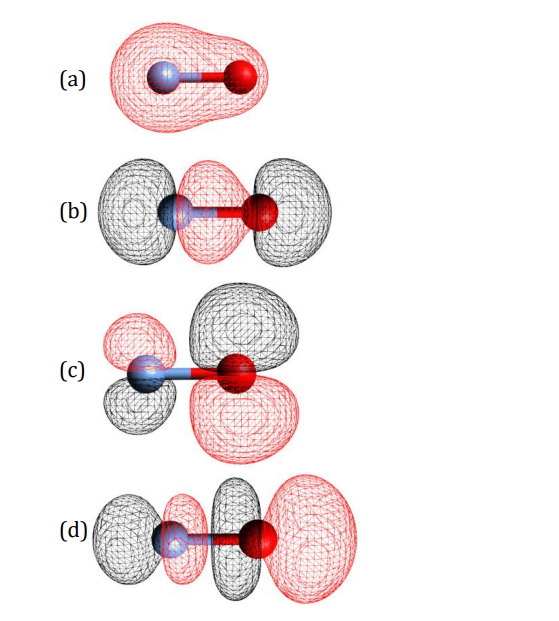
Transcribed Image Text:(а)
(b)
(с)
(d)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,