(e) Pure liquid styrene (phenylethene) is polymerized by adding a soluble peroxide initiator. When ial monomer concentration is 2.07 M and the peroxide concentration is 2 x 104 M, the initial polymerization (Vp) is 2.49 x 103 M min'. The rate constant for the decomposition of the er (k¡ ) is 5.7 x 10³ min'. termine the ratio kp/ (kt)*. State any assumptions. culate the expected number average molecular weight assuming termination takes place by mutual termination only.
(e) Pure liquid styrene (phenylethene) is polymerized by adding a soluble peroxide initiator. When ial monomer concentration is 2.07 M and the peroxide concentration is 2 x 104 M, the initial polymerization (Vp) is 2.49 x 103 M min'. The rate constant for the decomposition of the er (k¡ ) is 5.7 x 10³ min'. termine the ratio kp/ (kt)*. State any assumptions. culate the expected number average molecular weight assuming termination takes place by mutual termination only.
Chapter13: Kinetic Methods
Section: Chapter Questions
Problem 3P
Related questions
Question
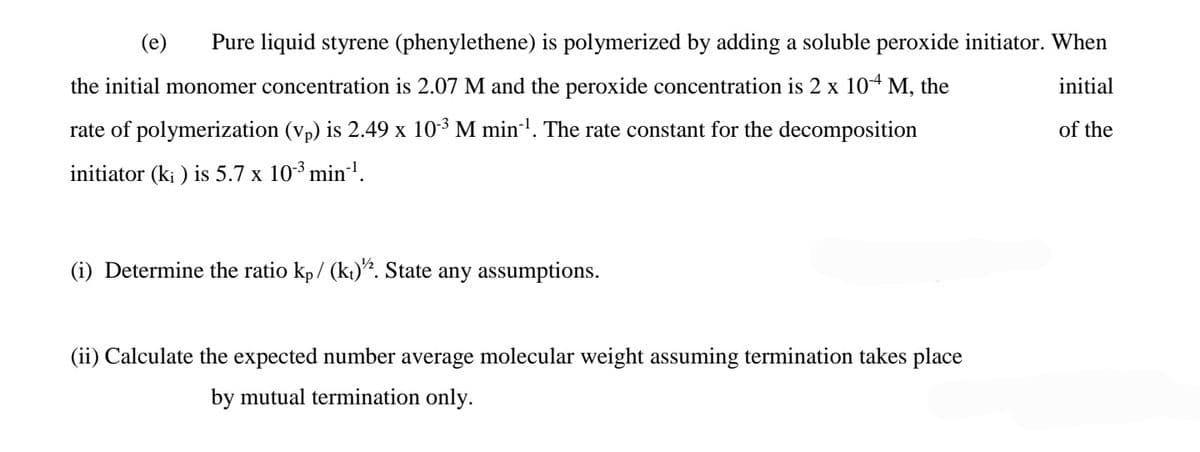
Transcribed Image Text:(e)
Pure liquid styrene (phenylethene) is polymerized by adding a soluble peroxide initiator. When
the initial monomer concentration is 2.07 M and the peroxide concentration is 2 x 104 M, the
initial
rate of polymerization (vp) is 2.49 x 103 M min . The rate constant for the decomposition
of the
initiator (k; ) is 5.7 x 103 min!.
(i) Determine the ratio kp/ (kt). State any assumptions.
(ii) Calculate the expected number average molecular weight assuming termination takes place
by mutual termination only.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,