Each of the following sets of quantun numbers is supposed to specify an crbitel, Hovever each sset contains one quantum nurber that Is rot allowed Reo ace the quantam sumber that is not allowed with one that is alowed (Eact change indicated in red) Part A n= 4, 1= 4, m =+3 O n=4, 1=3, m = +3 C n-4, 1-4, m -0 C n-3, 1= 4, m = +3 On= 4, 1=4, m - +4 Submit Request Answer Part B 25/ M P Type here to search 3/29/2021
Each of the following sets of quantun numbers is supposed to specify an crbitel, Hovever each sset contains one quantum nurber that Is rot allowed Reo ace the quantam sumber that is not allowed with one that is alowed (Eact change indicated in red) Part A n= 4, 1= 4, m =+3 O n=4, 1=3, m = +3 C n-4, 1-4, m -0 C n-3, 1= 4, m = +3 On= 4, 1=4, m - +4 Submit Request Answer Part B 25/ M P Type here to search 3/29/2021
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter6: Electronic Structure And Periodic Properties Of Elements
Section: Chapter Questions
Problem 34E: Answer the following questions: (a) Without using quantum numbers, describe the differences between...
Related questions
Question
100%
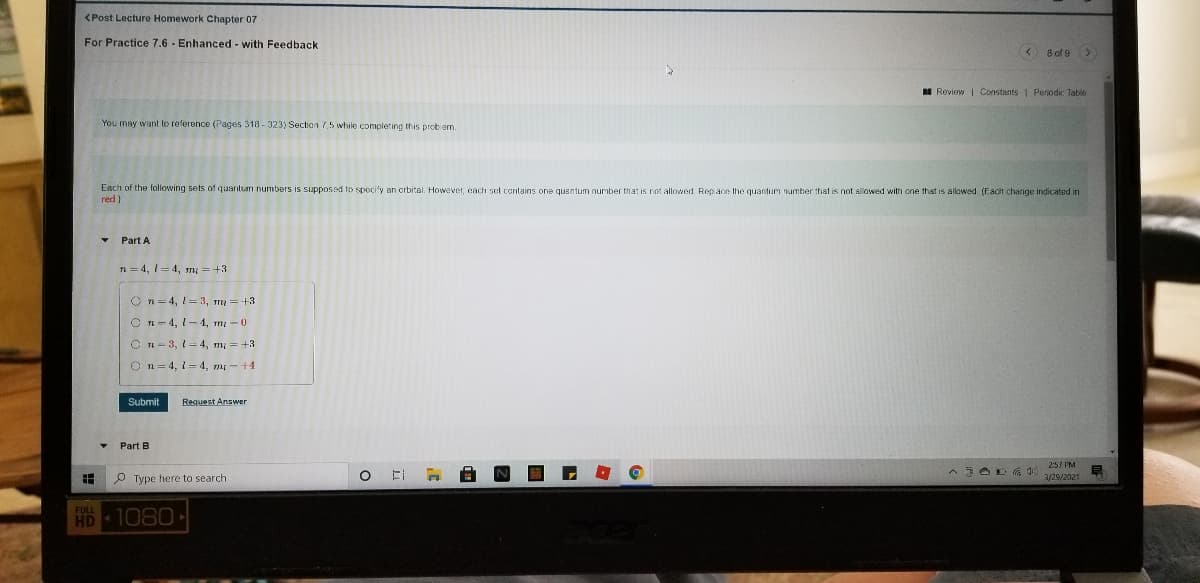
Transcribed Image Text:<Post Lecture Homework Chapter 07
For Practice 7.6 - Enhanced - with Feedback
8 of 9 >
I Reviow| Constants Periodic Table
You may want to reference (Pages 318 - 323) Section 75 while completing this prob em.
Each of the following sets of quantum numbers is supposed to specify an orbital. However, each set contains one quantum number that is not allowed, Rep ace the quanturm number that is not allowed with one that is alowed (Each change indicated in
red)
• Part A
n = 4, 1= 4, n =+3
O n= 4, 1= 3, m =+3
C n- 4, 1-4, m-0
O n- 3, 1=4, m = +3
O n= 4, 1=4, m -+4.
Submit
Reguest Answer
Part B
2:57 PM
O Type here to search
3/29/2021
FULL
HD 1080
LI
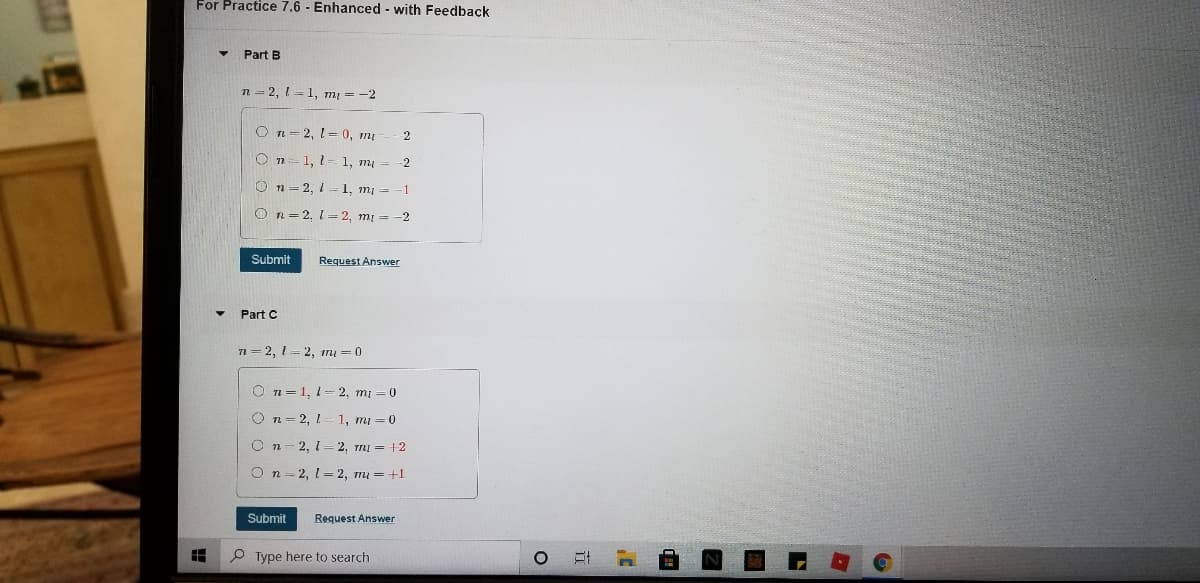
Transcribed Image Text:For Practice 7.6 - Enhanced - with Feedback
Part B
n = 2, 1 =1, mı = -2
O n= 2, 1= 0, mų
O n= 1, 1
1, m =
-2
O n= 2, 1 1, mi =
O n= 2, 1= 2, mị = -2
Submit
Request Answer
Part C
n = 2, 1 = 2, m = 0
O n=1, 1= 2, mi =0
O n= 2, L
1, mi = 0
O n
2, 1-2, TmI = +2
O n - 2, l= 2, mu = +1
Submit
Request Answer
P Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning