Each of the insoluble salts below are put into 0.10 M hydrochloric acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? Clear All iron(II) carbonate More soluble than in pure water. Similar solubility as in pure lead chloride water. Less soluble than in pure manganese(II) hydroxide water.
Each of the insoluble salts below are put into 0.10 M hydrochloric acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? Clear All iron(II) carbonate More soluble than in pure water. Similar solubility as in pure lead chloride water. Less soluble than in pure manganese(II) hydroxide water.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter15: Complex Ion And Precipitation Equilibria
Section: Chapter Questions
Problem 63QAP: Marble is almost pure CaCO3. Acid rain has a devastating effect on marble statuary left outdoors....
Related questions
Question
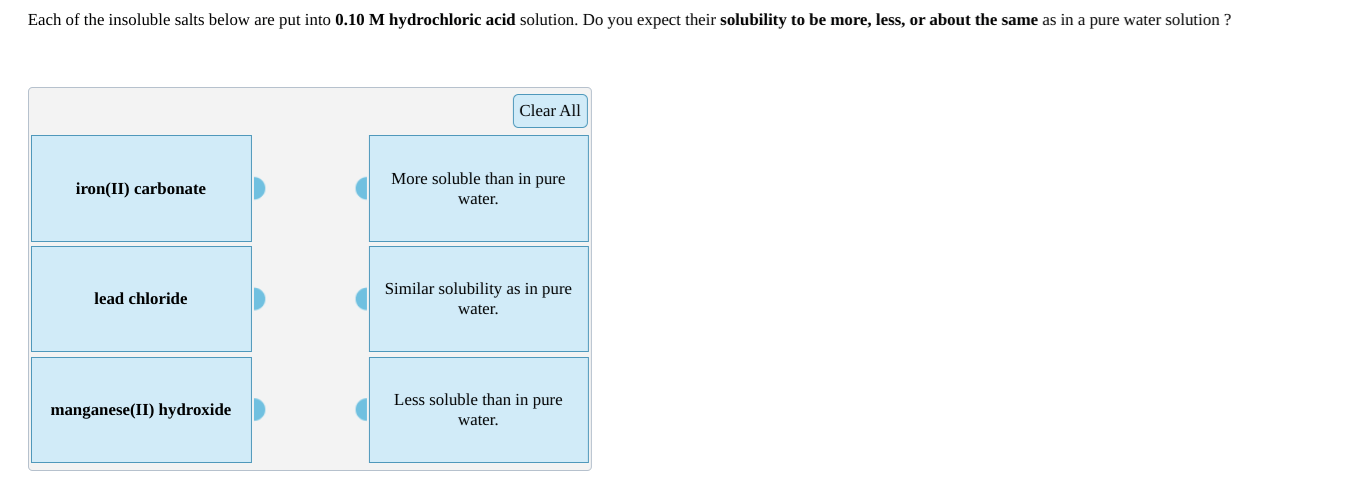
Transcribed Image Text:Each of the insoluble salts below are put into 0.10 M hydrochloric acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ?
Clear All
iron(II) carbonate
More soluble than in pure
water.
Similar solubility as in pure
lead chloride
water.
Less soluble than in pure
manganese(II) hydroxide
water.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning