Electrode 1 Cu Cu Cu Cu Zn Zn Zn Pb Pb Mg Electrode 2 Zn Pb Mg Sn Pb Mg Sn Mg Sn Sn Cell potential Ecell in V 0 O 0.9% Zn Сн 0401 v Pb Cu Cu 1o 480 mg •363 Sn 515 20 G Anode 692 482 2n Mg Bo 10121 Cathode + Ecell = Ecathode - E anode Cu рь 2n Sn рь 16-17 pb 1113 Mg Sn Substance Substance Oxidized = Reducing Agent Reduced= Oxidizing Agent Cutz Zn +2 Pb Cu Cutz Ma +2 +2 Cutz Sn +2 2n 42 2n² Mg +2 2n pb Sn +2 Pb mg +2 Sn² Pb 'n +2 Sn mg Postlab #1: E cathode Postlab #1: E anode Postlab #1: Eº cell
Electrode 1 Cu Cu Cu Cu Zn Zn Zn Pb Pb Mg Electrode 2 Zn Pb Mg Sn Pb Mg Sn Mg Sn Sn Cell potential Ecell in V 0 O 0.9% Zn Сн 0401 v Pb Cu Cu 1o 480 mg •363 Sn 515 20 G Anode 692 482 2n Mg Bo 10121 Cathode + Ecell = Ecathode - E anode Cu рь 2n Sn рь 16-17 pb 1113 Mg Sn Substance Substance Oxidized = Reducing Agent Reduced= Oxidizing Agent Cutz Zn +2 Pb Cu Cutz Ma +2 +2 Cutz Sn +2 2n 42 2n² Mg +2 2n pb Sn +2 Pb mg +2 Sn² Pb 'n +2 Sn mg Postlab #1: E cathode Postlab #1: E anode Postlab #1: Eº cell
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 48P
Related questions
Question
100%
I am trying to figure out how to fill in the last part of that chart that is supposed to come from What I did in the measured cell potential

Transcribed Image Text:. (12) In Part I of the procedure, you measured the cell potential for a variety of combinations of
half cells. Use a standard reduction potential table in your textbook to calculate the expected
Eᵒcell using the formula Eºcell = Eºcathode - - E°anode. Calculate Eºcell for each combination
of cells. Show work for one calculation here, but you can enter the values in the table on page
6.
M = 0.581
S 8.99
1000
5.81
100
58.99
Actual concentration of conce
١٨ ٥٠٠ من one لسور
Ma
m-0098
0.098 but the
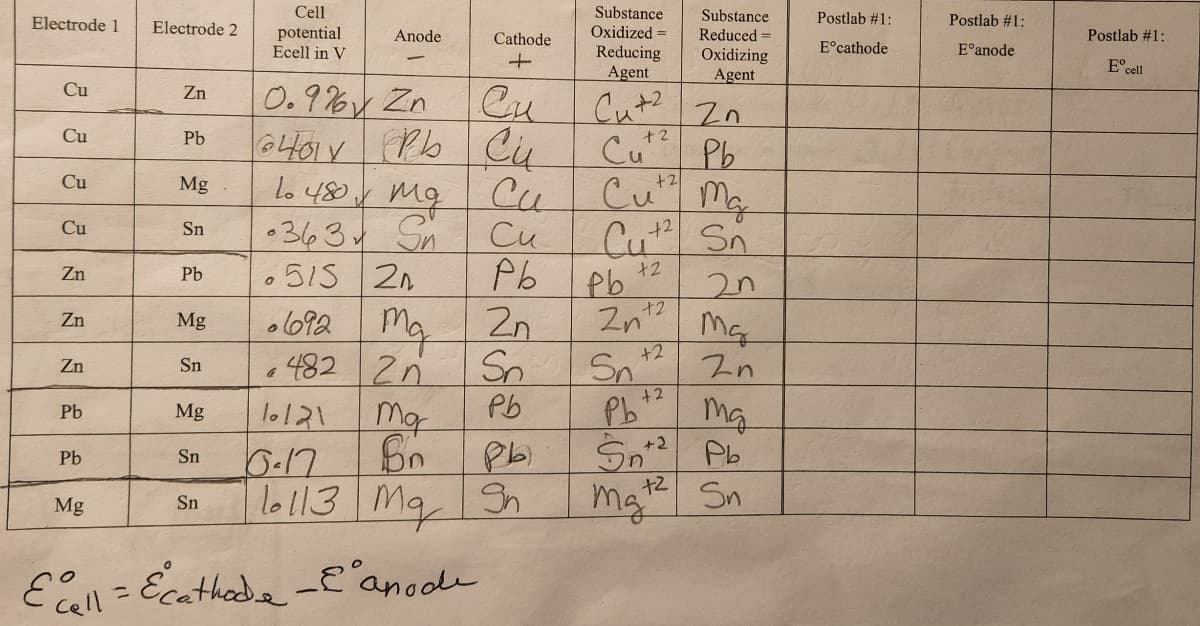
Transcribed Image Text:Electrode 1
Cu
Cu
Cu
Cu
Zn
Zn
Zn
Pb
Pb
Mg
Electrode 2
Zn
Pb
Mg
Sn
Pb
Mg
Sn
Mg
Sn
Sn
Cell
potential
Ecell in V
0.976 Zn
Cai
оных трь си
10 480X
си
0
Anode
mg
•363 S Cu
515 2n
рь
2n
.692
482 2n
Ma
Bo
10/21
Cathode
Ecell = Ecathode - E anode
Sp
рь
16-17
pb
10113 Ma Sh
Substance
Oxidized =
Reducing
Agent
Zn
+2
Cu+2
Cut Pb
Cutz Ma
Cutz Sn
+2
Pb
42
2n+2
+2
Sn
+2
Pb ¹²
Substance
Reduced=
Oxidizing
Agent
mg
2n
Mg
2n
mg
+2
Sn2 Pb
+2
Sn
Postlab #1:
Eᵒcathode
Postlab #1:
E anode
Postlab #1:
Eºcell
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
