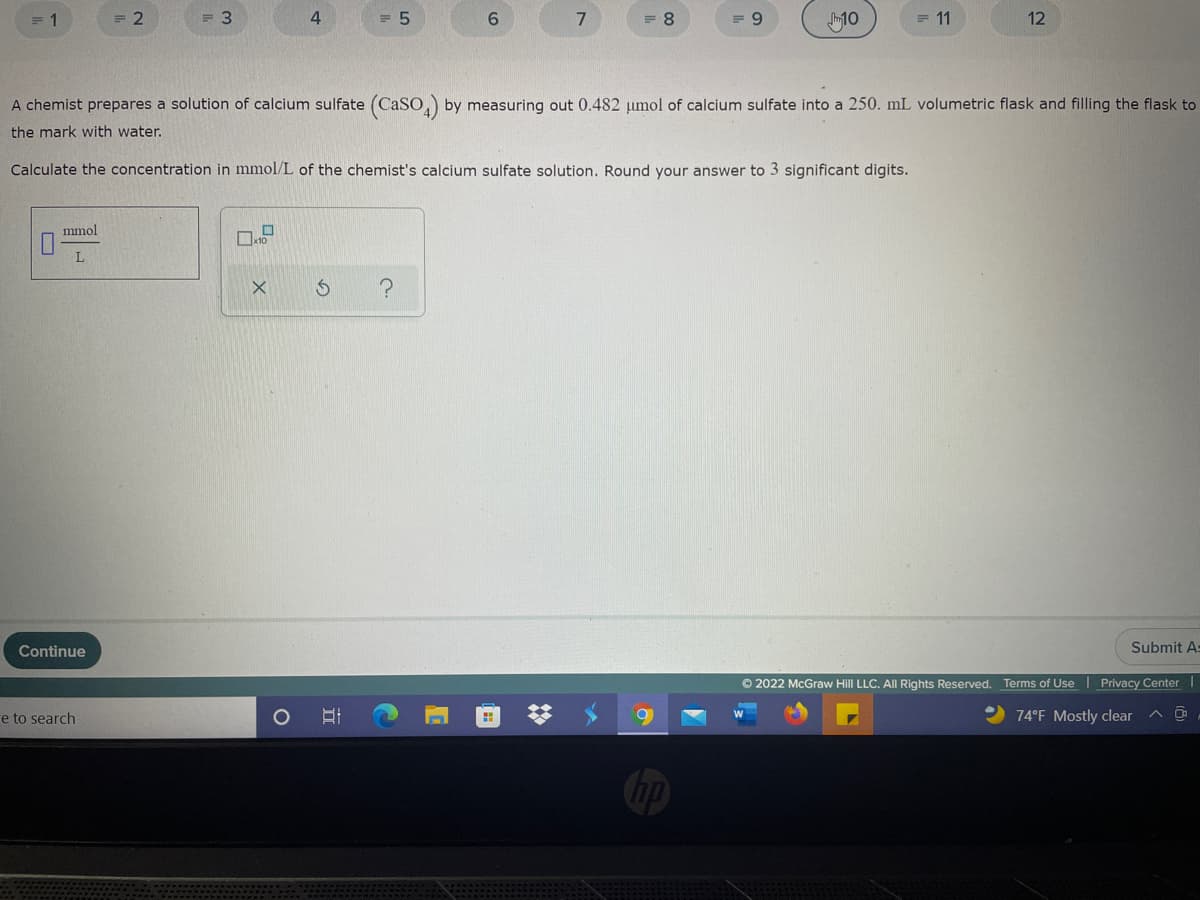
emist prepares a solution of calcium sulfate (CaSO,) by measuring out 0.482 umol of calcium sulfate into a 250. mL volumetric flask and filling the flask to mark with water. ulate the concentration in mmol/L of the chemist's calcium sulfate solution. Round your answer to 3 significant digits. mmol L.
emist prepares a solution of calcium sulfate (CaSO,) by measuring out 0.482 umol of calcium sulfate into a 250. mL volumetric flask and filling the flask to mark with water. ulate the concentration in mmol/L of the chemist's calcium sulfate solution. Round your answer to 3 significant digits. mmol L.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.51QE
Related questions
Question
Transcribed Image Text:= 1
= 2
= 3
= 5
6.
7
= 8
10
= 11
12
A chemist prepares a solution of calcium sulfate (CaSo,) by measuring out 0.482 umol of calcium sulfate into a 250. mL volumetric flask and filling the flask to
the mark with water.
Calculate the concentration in mmol/L of the chemist's calcium sulfate solution. Round your answer to 3 significant digits.
mmol
Continue
Submit A=
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center
e to search
74°F Mostly clear
立
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning