Enter a balanced chemical equation for the fermentation of glucose (C6H1206) by Clostridium pasteurianum in which the aqueous sugar reacts with water to form aqueous acetic acid (CH3CO,H), carbonic acid (H2CO3), and hydrogen gas. Express your answer as a chemical equation including phases.
Enter a balanced chemical equation for the fermentation of glucose (C6H1206) by Clostridium pasteurianum in which the aqueous sugar reacts with water to form aqueous acetic acid (CH3CO,H), carbonic acid (H2CO3), and hydrogen gas. Express your answer as a chemical equation including phases.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter2: Matter And Energy
Section: Chapter Questions
Problem 7E: 7.The word pour is commonly used in reference to liquids but not to solids or gases. Can you pour a...
Related questions
Question
Question in Image.
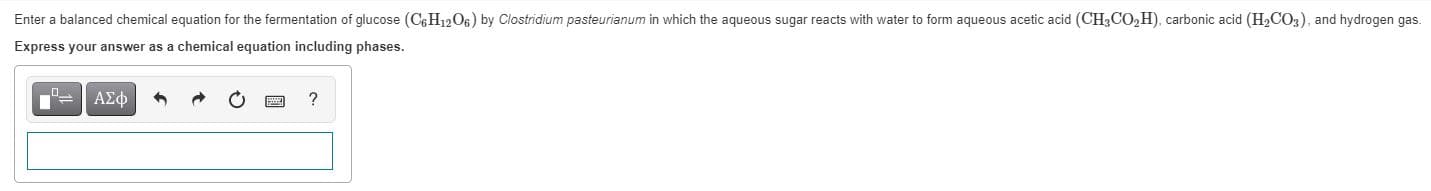
Transcribed Image Text:Enter a balanced chemical equation for the fermentation of glucose (C6H1206) by Clostridium pasteurianum in which the aqueous sugar reacts with water to form aqueous acetic acid (CH3CO,H), carbonic acid (H2CO3), and hydrogen gas.
Express your answer as a chemical equation including phases.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning
