Chapter7: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 139AE: Mars is roughly 60 million km from the earth. How long does it take for a radio signal originating...
Related questions
Question
100%
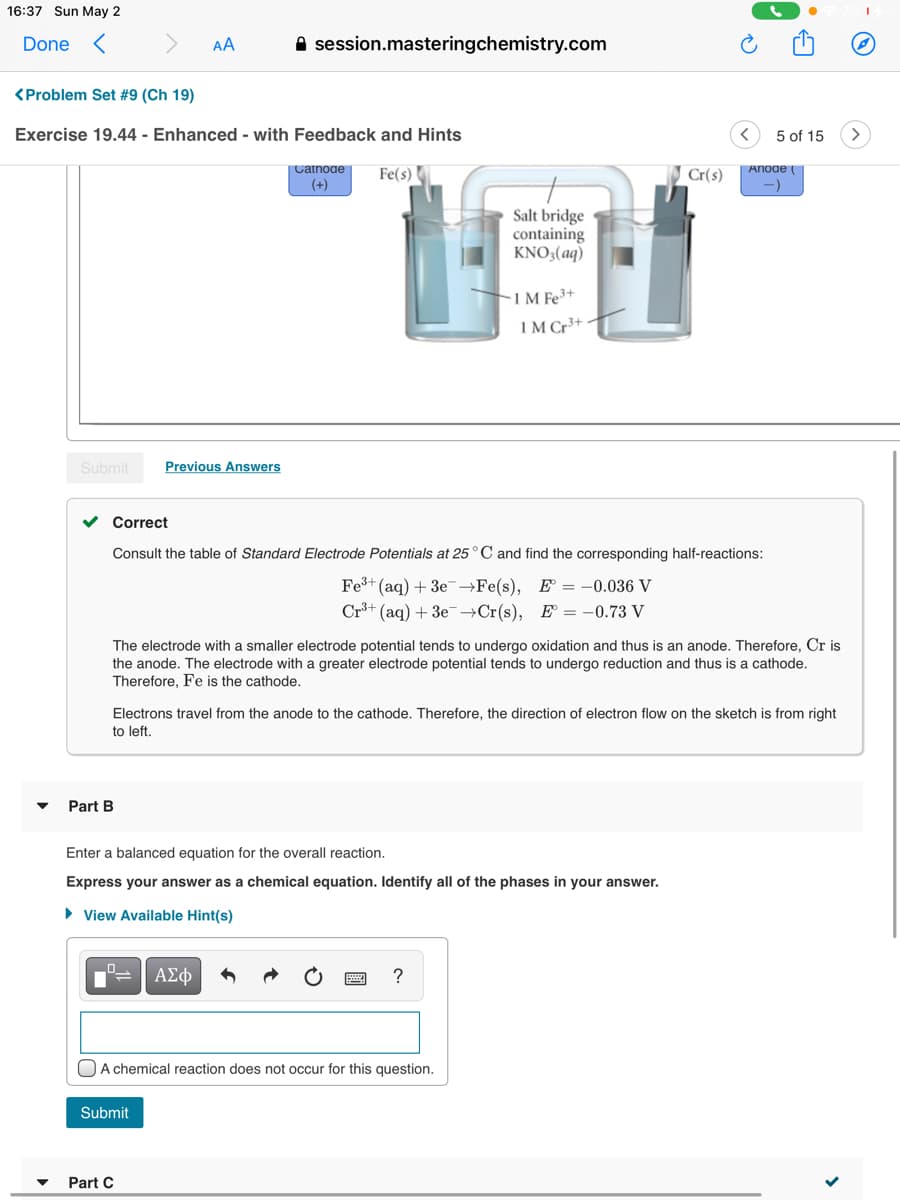
Transcribed Image Text:16:37 Sun May 2
Done <
AA
A session.masteringchemistry.com
<Problem Set #9 (Ch 19)
Exercise 19.44 - Enhanced - with Feedback and Hints
5 of 15
Catnoae
(+)
Anoae
Fe(s)
Cr(s)
Salt bridge
containing
KNO3(aq)
-1 M Fe3+
IM Cr³+
Submit
Previous Answers
Correct
Consult the table of Standard Electrode Potentials at 25° C and find the corresponding half-reactions:
Fe3+ (ag) + 3e-→Fe(s), E = -0.036 V
Cr+ (aq) + 3e-→Cr(s), E = -0.73 V
The electrode with a smaller electrode potential tends to undergo oxidation and thus is an anode. Therefore, Cr is
the anode. The electrode with a greater electrode potential tends to undergo reduction and thus is a cathode.
Therefore, Fe is the cathode.
Electrons travel from the anode to the cathode. Therefore, the direction of electron flow on the sketch is from right
to left.
Part B
Enter a balanced equation for the overall reaction.
Express your answer as a chemical equation. Identify all of the phases in your answer.
• View Available Hint(s)
ΑΣφ
?
O A chemical reaction does not occur for this question.
Submit
Part C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning