enter formula 1) The strongest oxidizing agent is: (2) The weakest oxidizing agent is: (3) The weakest reducing agent is: (4) The strongest reducing agent is:
enter formula 1) The strongest oxidizing agent is: (2) The weakest oxidizing agent is: (3) The weakest reducing agent is: (4) The strongest reducing agent is:
Chapter22: Bulk Electrolysis: Electrogravimetry And Coulometry
Section: Chapter Questions
Problem 22.28QAP
Related questions
Question
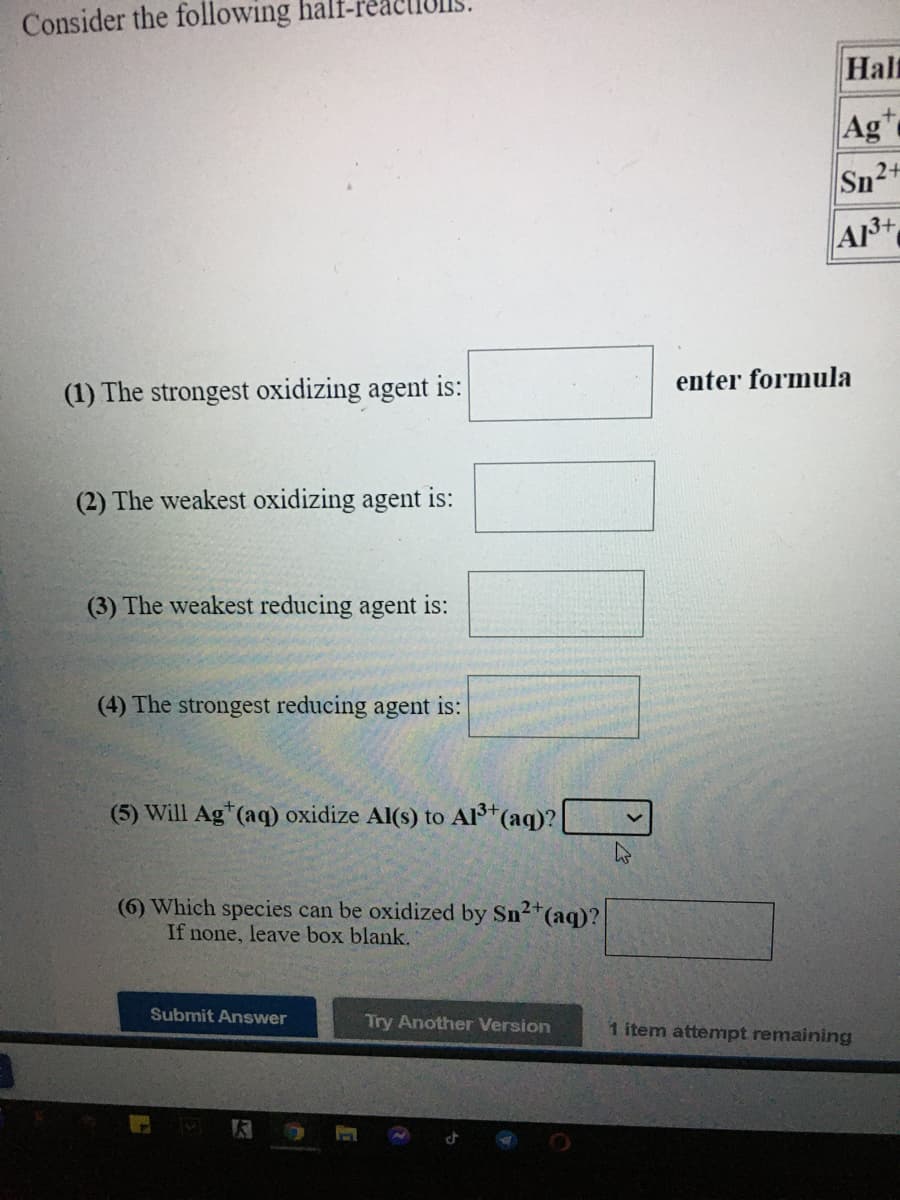
Transcribed Image Text:Consider the following half-r
Hal
Ag
Sn2+
A3+
(1) The strongest oxidizing agent is:
enter formula
(2) The weakest oxidizing agent is:
(3) The weakest reducing agent is:
(4) The strongest reducing agent is:
(5) Will Ag*(aq) oxidize Al(s) to Al(aq)?
(6) Which species can be oxidized by Sn2*(aq)?
If none, leave box blank.
Submit Answer
Try Another Version
1 item attempt remaining
![lm/takeAssignment/takeCovalentActivity.do?locator=Dassignment-take
[References]
Use the References to access important values if needed for th
Consider the following half-reactions:
Half-reaction
E° (V)
Ag" (aq) + e-
Ag(s) 0.799V
2+
Sn-"(aq) + 2e Sn(s) -0.140V
Al3+
"(аq) + Зе
→ Al(s) -1.660V
(1) The strongest oxidizing agent is:
enter formula
(2) The weakest oxidizing agent is:
(3) The weakest reducing agent is:](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F8e22edbd-5826-4ddc-b445-745b0c721354%2F852f286f-fd11-4ed8-9e91-c6f6771d3fb4%2Fr3s05pr_processed.jpeg&w=3840&q=75)
Transcribed Image Text:lm/takeAssignment/takeCovalentActivity.do?locator=Dassignment-take
[References]
Use the References to access important values if needed for th
Consider the following half-reactions:
Half-reaction
E° (V)
Ag" (aq) + e-
Ag(s) 0.799V
2+
Sn-"(aq) + 2e Sn(s) -0.140V
Al3+
"(аq) + Зе
→ Al(s) -1.660V
(1) The strongest oxidizing agent is:
enter formula
(2) The weakest oxidizing agent is:
(3) The weakest reducing agent is:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning