Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter12: Alkenes And Alkynes
Section: Chapter Questions
Problem 12.42P: 12-42 Complete these equations.
Related questions
Question
Enter in order the abbreviations for the electron geometry (EG) and coordination number (CN) of the central atom for each substance
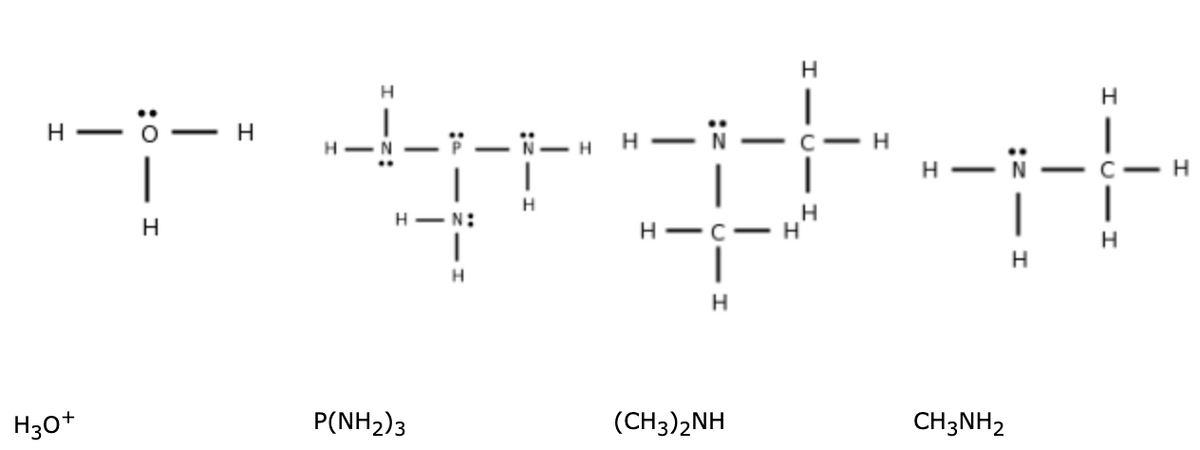
Transcribed Image Text:H
H
H
H - 0 -
H-N -
-
C- H
H
C -
H
H
H - N:
H30+
P(NH2)3
(CH3)2NH
CH3NH2
I -U -I
:z - I
:0 - I
Expert Solution
Step 1
The expression for calculating the coordination number is shown below:
Step 2
The structure of given compound is shown below:
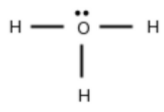
The calculation of coordination number of central atom is shown below:
In electronic geometry; lone pair also considered. So; the electron geometry of H3O+ is tetrahedral.
Step 3
The structure of given compound is shown below:
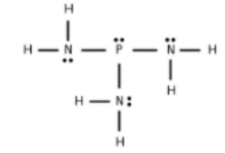
The calculation of coordination number of central atom is shown below:
In electronic geometry; lone pair also considered. So; the electron geometry of P(NH2)3 is tetrahedral.
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning