enthalpy of steam at 155 deg
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
What is the enthalpy of steam at 155 deg C?
Find the specific enthalpy of superheated steam at 2 atm and 900°C.
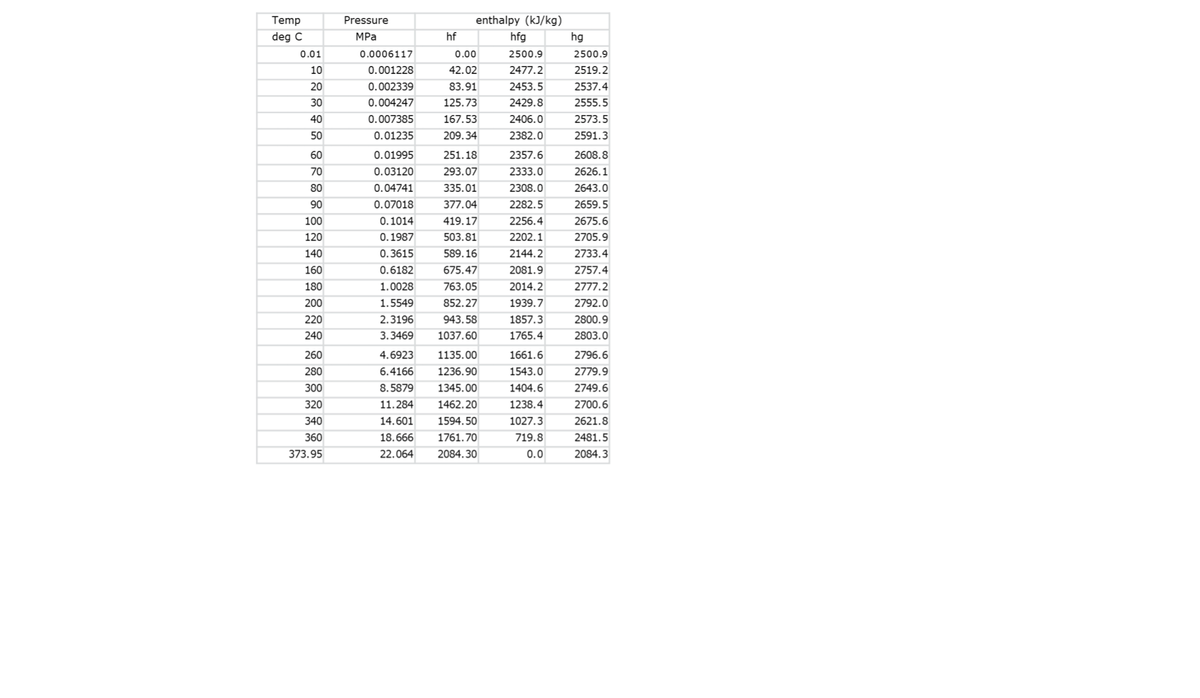
Transcribed Image Text:Temp
Pressure
enthalpy (kJ/kg)
deg C
MPа
hf
hfg
hg
0.01
0.0006117
0.00
2500.9
2500.9
10
0.001228
42.02
2477.2
2519.2
20
0.002339
83.91
2453.5
2537.4
30
0.004247
125.73
2429.8
2555.5
40
0.007385
167.53
2406.0
2573.5
50
0.01235
209.34
2382.0
2591.3
60
0.01995
251.18
2357.6
2608.8
70
0.03120
293.07
2333.0
2626.1
80
0.04741
335.01
2308.0
2643.0
90
0.07018
377.04
2282.5
2659.5
100
0.1014
419.17
2256.4
2675.6
120
0.1987
503.81
2202.1
2705.9
140
0.3615
589.16
2144.2
2733.4
160
0.6182
675.47
2081.9
2757.4
180
1.0028
763.05
2014.2
2777.2
200
1.5549
852.27
1939.7
2792.0
220
2.3196
943.58
1857.3
2800.9
240
3.3469
1037.60
1765.4
2803.0
260
4.6923
1135.00
1661.6
2796.6
280
6.4166
1236.90
1543.0
2779.9
300
8.5879
1345.00
1404.6
2749.6
320
11.284
1462.20
1238.4
2700.6
340
14.601
1594. 50
1027.3
2621.8
360
18.666
1761.70
719.8
2481.5
373.95
22.064
2084.30
0.0
2084.3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY