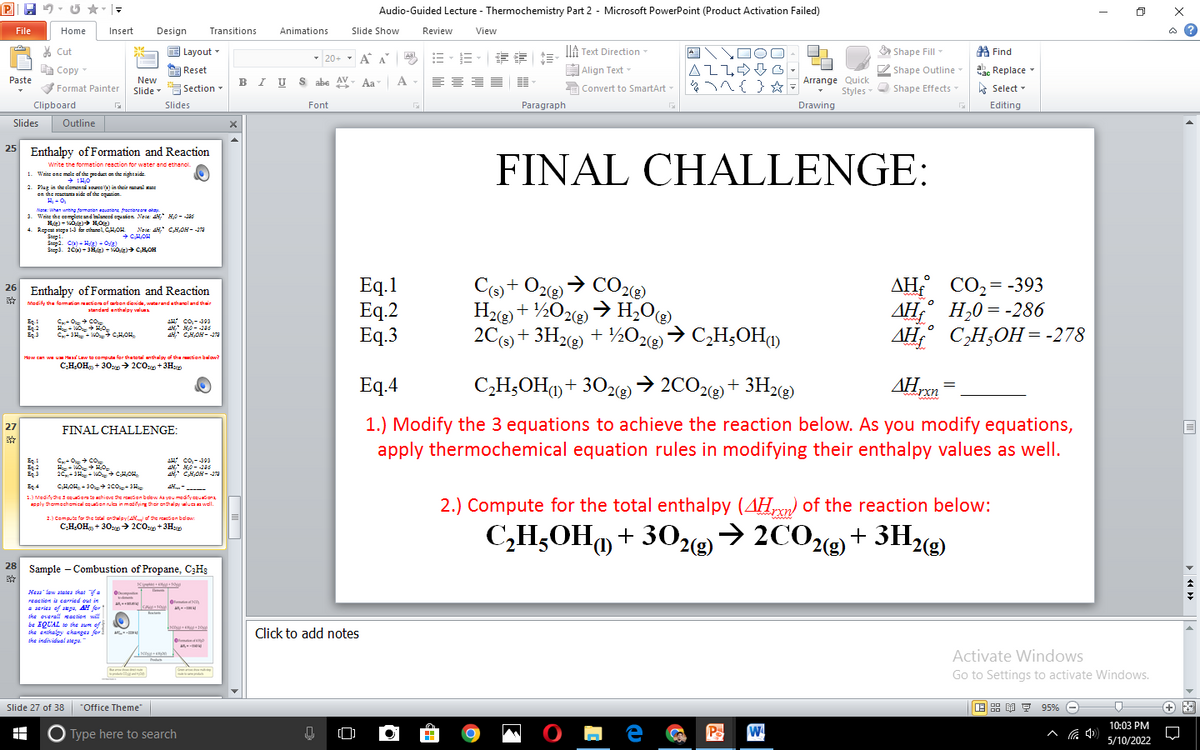
Eq.1 Eq.2 Eq.3 C9)+ O2@)→ CO2@) H22) + ½O2@ → H,O® 2C(s) + 3Hg) + ½O2«g→ C¸H;OH AH CO2=-393 AH,° H,0= -286 ДН. СНОН%3-278 Eq.4 C,H;OH) + 302g→ 2CO22)+ 3H2(g) AHrxn 1.) Modify the 3 equations to achieve the reaction below. As you modify equations, apply thermochemical equation rules in modifying their enthalpy values as well. 2.) Compute for the total enthalpy (4Hm) of the reaction below: C,H;OH + 30z«e→ 2COg) + 3H2)
Eq.1 Eq.2 Eq.3 C9)+ O2@)→ CO2@) H22) + ½O2@ → H,O® 2C(s) + 3Hg) + ½O2«g→ C¸H;OH AH CO2=-393 AH,° H,0= -286 ДН. СНОН%3-278 Eq.4 C,H;OH) + 302g→ 2CO22)+ 3H2(g) AHrxn 1.) Modify the 3 equations to achieve the reaction below. As you modify equations, apply thermochemical equation rules in modifying their enthalpy values as well. 2.) Compute for the total enthalpy (4Hm) of the reaction below: C,H;OH + 30z«e→ 2COg) + 3H2)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section: Chapter Questions
Problem 16.CCP
Related questions
Question
What is the answer to this question? Thank you!
Transcribed Image Text:Audio-Guided Lecture - Thermochemistry Part 2 - Microsoft PowerPoint (Product Activation Failed)
File
Home
Insert
Design
Transitions
Animations
Slide Show
Review
View
* Cut
Layout -
IA Text Direction
Shape Fill-
第Find
Aal
20+ - A A E- E
E Copy
A Reset
Align Text -
AZLOB
Shape Outline
ab. Replace
BI U S abe AV- Aa
A -
Paste
New
Arrange Quick
Slide- "G Section-
Slides
Format Painter
E Convert to SmartArt-
Styles- Shape Effects-
A Select -
Clipboard
Font
Paragraph
Drawing
Editing
Slides
Outline
25 Enthalpy of Formation and Reaction
FINAL CHALLENGE:
Write the formation reaction for water and ethanol.
1. Waite ene mele ef the peda on the right side
2. Plug in theclementi 1oea) in their tal st:
en the etatide ef the eqanion
.ahay םוב גרמ םדמיבבבה הבליבודוב,תמrואי המבאל
3. Write the cemplete and laneed eguation Neie AH, H0- -2as
Nate: Whan untng famatan aata frecton re oba
Nase AH, CHOH- J
+ CHOH
4. Ropeat atopa a le eshanel CHOH
Step.
Step2. Ca) - H/3) - 0/3)
Seepa. 20) - 3H) - VOg) CHOH
Eq.1
Eq.2
Eq.3
Co + O2 > CO22)
H2(g) + ½02(g)
2C + 3Hz + ½O2→ C,H;OH
AH CO,= -393
ДН
AH, C,H,OH=-278
26 Enthalpy of Formation and Reaction
Medify the fomation tiom of arten diexide, waterandatharcland thein
atandard anthalpy valu
AH
Н,0 %3D -286
CO CO
AH co.-403
Eq.!
Eg2
Eg
A CHON- 1
How can we um Heaa Lw to computa for thatotal nthaly of the uction below?
CH,OH + 30:> 2C0+3H
Eq.4
C,H,OH() + 302@) → 2CO2(2) + 3H2@)
AHrn
www
1.) Modify the 3 equations to achieve the reaction below. As you modify equations,
apply thermochemical equation rules in modifying their enthalpy values as well.
27
FINAL CHALLENGE:
CO CO
AH Co.-393
20-3- WO CHOH
AH CHOH -
Eg.4
CHOH - 30 200H
-----
1.) Medify dhe s cquiom to achiove dhe reacion beow Aa you moafycguationa
ssly thomechomical cqudion nula in madilying dhair enthalpy waluOa wel.
2.) Compute for the total enthalpy (AHrm) of the reaction below:
z.) Compute for the total onthalpy(4H the reation bdow:
2COe + 3H2(g)
C,HOH, + 30y> 2C0 +3Hy
C,H;OH + 302(>
28
Sample - Combustion of Propane, C3H3
reaction a cerried out in
* seriea of aka, AH for
ske overall eaction sill
be IQUAL o ske um of
ske enikaly ekanges for
ske individual ataga.
Click to add notes
Activate Windows
Go to Settings to activate Windows.
Slide 27 of 38
"Office Theme"
9 95%
10:03 PM
O Type here to search
^ G 4)
5/10/2022
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning