Equilibrium reaction: Co(H₂O)²+ + 4C1 CoCl² + 6H₂O In the virtual lab the colors below represent the concentrations of reactants and products in the solution Pink means: More reactants at equilibrium Blue means: More products at equilibrium Purple means: Equal concentrations at equilibrium
Equilibrium reaction: Co(H₂O)²+ + 4C1 CoCl² + 6H₂O In the virtual lab the colors below represent the concentrations of reactants and products in the solution Pink means: More reactants at equilibrium Blue means: More products at equilibrium Purple means: Equal concentrations at equilibrium
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 87AP: . Many sugars undergo a process called mutarotation, in which the sugar molecules interconvert...
Related questions
Question
100%
![11:14 PM Sun Jul 3
FLVS
AA
1 VG HELP Site-C... A7.06.mp4 -...
MENU
B] (07.05 HC) I...
learn.flvs.net
Lessons Assessments Gradebook
Co(H₂O) 6²+ + 4CI CoCl² + 6H₂O
11 V18: 4766
JOURNALS GLOSSARY PRINT TOOLKIT OBJECTIVES
CONTROL
Email 10
SHOW TEXT VERSION
Tools
You will investigate an equilibrium system between two cobalt complex ions.
How does a...
CONTINUE
7 of 7 ▼
x 1 V18: 4766
In aqueous solution, Co(H₂O) 62+ is pink, CoCl4²- is blue, and CI and H₂O are
colorless. The distinctive colors of the two cobalt (II) species in solution provide a visual
comparison of concentrations. This allows you to observe how the concentrations of the
two cobalt complex ions compare within the system.
My Courses
HOME
58% (
00
00
★ Start Page
TALIA ▾](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fce8ff09d-d7fb-40b0-952f-4b5e878d8451%2F69b31528-4486-47cd-af53-8b1955ec18a0%2Fb0t5q5m_processed.jpeg&w=3840&q=75)
Transcribed Image Text:11:14 PM Sun Jul 3
FLVS
AA
1 VG HELP Site-C... A7.06.mp4 -...
MENU
B] (07.05 HC) I...
learn.flvs.net
Lessons Assessments Gradebook
Co(H₂O) 6²+ + 4CI CoCl² + 6H₂O
11 V18: 4766
JOURNALS GLOSSARY PRINT TOOLKIT OBJECTIVES
CONTROL
Email 10
SHOW TEXT VERSION
Tools
You will investigate an equilibrium system between two cobalt complex ions.
How does a...
CONTINUE
7 of 7 ▼
x 1 V18: 4766
In aqueous solution, Co(H₂O) 62+ is pink, CoCl4²- is blue, and CI and H₂O are
colorless. The distinctive colors of the two cobalt (II) species in solution provide a visual
comparison of concentrations. This allows you to observe how the concentrations of the
two cobalt complex ions compare within the system.
My Courses
HOME
58% (
00
00
★ Start Page
TALIA ▾
Transcribed Image Text:7:46 PM Tue Jul 5
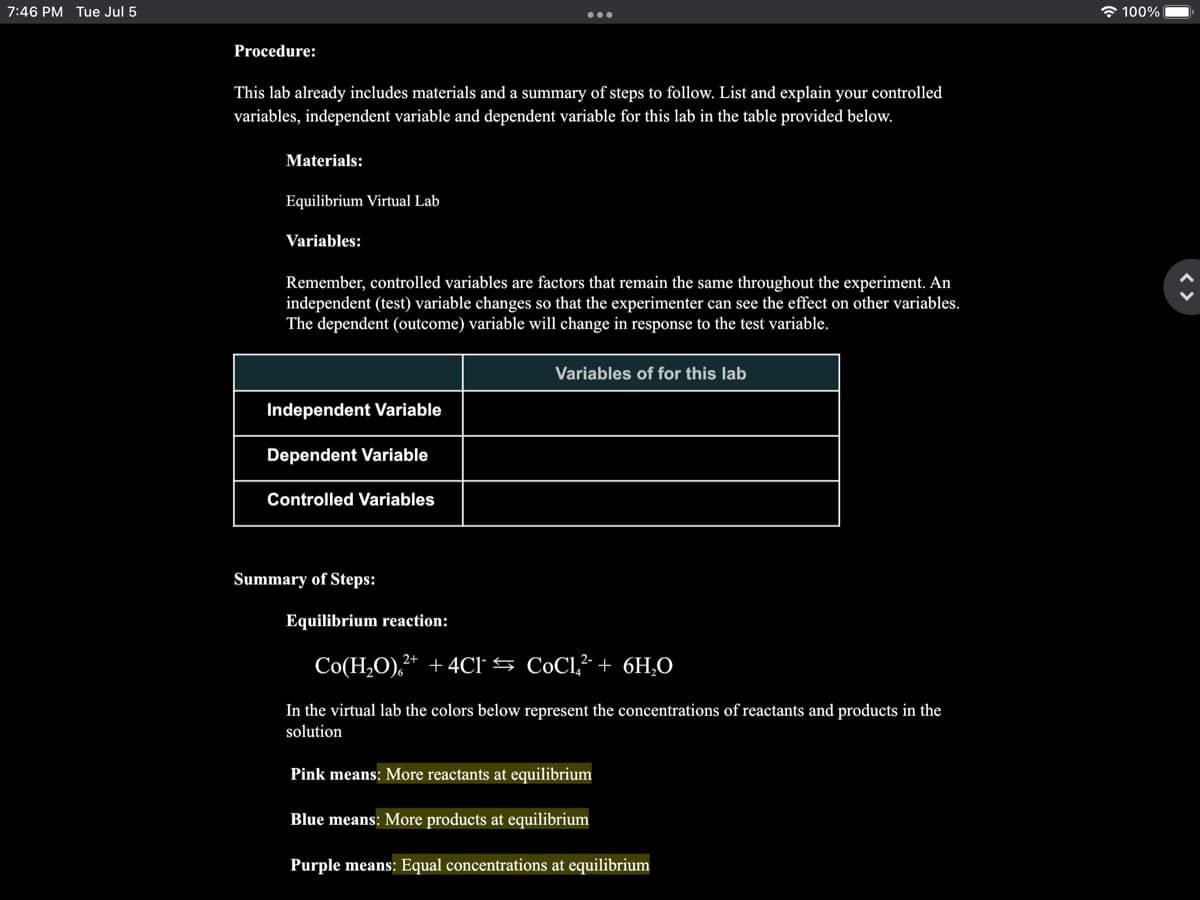
Procedure:
This lab already includes materials and a summary of steps to follow. List and explain your controlled
variables, independent variable and dependent variable for this lab in the table provided below.
Materials:
Equilibrium Virtual Lab
Variables:
Remember, controlled variables are factors that remain the same throughout the experiment. An
independent (test) variable changes so that the experimenter can see the effect on other variables.
The dependent (outcome) variable will change in response to the test variable.
Independent Variable
Dependent Variable
Controlled Variables
Summary of Steps:
Equilibrium reaction:
Variables of for this lab
Co(H₂O),²+ + 4C1
CoCl² + 6H₂O
In the virtual lab the colors below represent the concentrations of reactants and products in the
solution
Pink means: More reactants at equilibrium
Blue means: More products at equilibrium
Purple means: Equal concentrations at equilibrium
100%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning