Essential oils are concentrated liquid containing metabolites extracted from plant sources and are widely used in the production of perfumes. The structures of some of these compounds present in essential oils, along with acetone, are presented below. HgCO. HO CH b. C. H₂ Compound A MM: 164.2 g/mol CH₂ H₂C Compound B MM: 162.19 g/mol FCH₂ H₂C H₂C CH3 H₂C CH CH₂ CH₂ Compound C MM: 136.24 g/mol 1. Determine the most possible boiling point of each of the compounds. Refer to the possible boiling points (232°C, 254°C, 176°C) 2. An equal volume of COMPOUNDS A, B and C were stored in separate beakers which were left uncovered at room temperature. If the three liquids were allowed to stand for about 10 minutes, which of the following will have the least amount of liquid remaining in the container? a. What physical property is the basis of the answer? Among the three liquids, what is the most recommended ingredient of a perfume that will have the most lasting scent?
Essential oils are concentrated liquid containing metabolites extracted from plant sources and are widely used in the production of perfumes. The structures of some of these compounds present in essential oils, along with acetone, are presented below. HgCO. HO CH b. C. H₂ Compound A MM: 164.2 g/mol CH₂ H₂C Compound B MM: 162.19 g/mol FCH₂ H₂C H₂C CH3 H₂C CH CH₂ CH₂ Compound C MM: 136.24 g/mol 1. Determine the most possible boiling point of each of the compounds. Refer to the possible boiling points (232°C, 254°C, 176°C) 2. An equal volume of COMPOUNDS A, B and C were stored in separate beakers which were left uncovered at room temperature. If the three liquids were allowed to stand for about 10 minutes, which of the following will have the least amount of liquid remaining in the container? a. What physical property is the basis of the answer? Among the three liquids, what is the most recommended ingredient of a perfume that will have the most lasting scent?
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter3: Composition Of Substances And Solutions
Section: Chapter Questions
Problem 77E: Copper(I) iodide (CuI) is often added to table salt as a dietary source of iodine. How many moles of...
Related questions
Question
Please kindly answer numbers 1 and 2 (a. b. c.). Thank you!
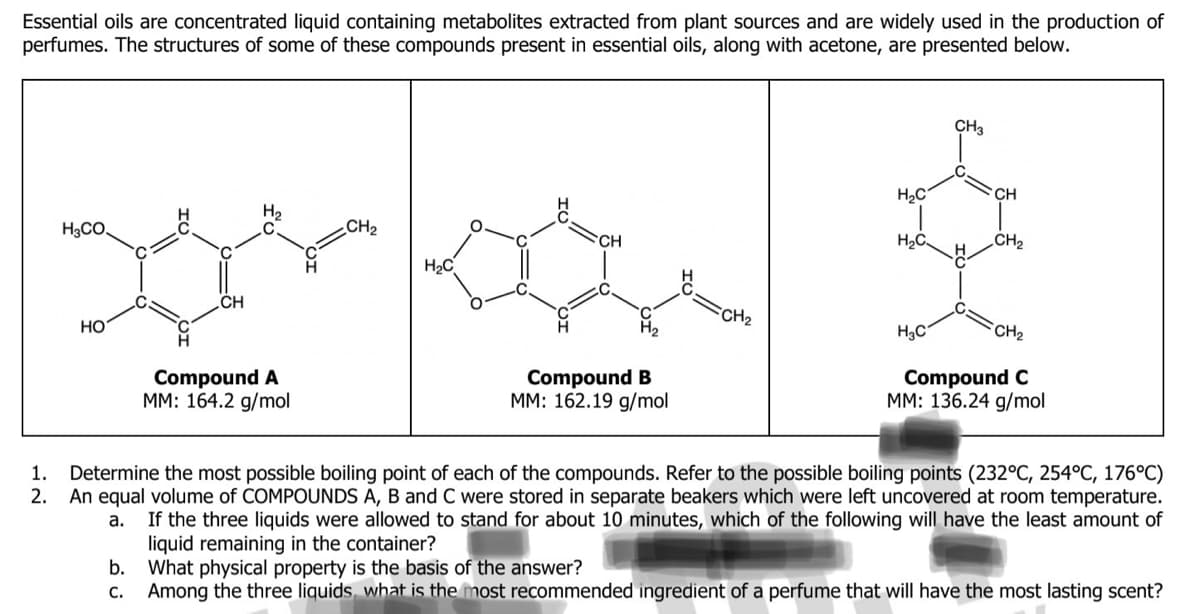
Transcribed Image Text:Essential oils are concentrated liquid containing metabolites extracted from plant sources and are widely used in the production of
perfumes. The structures of some of these compounds present in essential oils, along with acetone, are presented below.
1.
2.
H3CO.
HO
CH
b.
C.
Compound A
MM: 164.2 g/mol
CH₂
H₂C
Compound B
MM: 162.19 g/mol
FCH₂
H₂C
H₂C
CH3
H₂C
CH
CH₂
CH₂
Compound C
MM: 136.24 g/mol
Determine the most possible boiling point of each of the compounds. Refer to the possible boiling points (232°C, 254°C, 176°C)
An equal volume of COMPOUNDS A, B and C were stored in separate beakers which were left uncovered at room temperature.
If the three liquids were allowed to stand for about 10 minutes, which of the following will have the least amount of
liquid remaining in the container?
a.
What physical property is the basis of the answer?
Among the three liquids, what is the most recommended ingredient of a perfume that will have the most lasting scent?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax