et al. 2004; Yu et al. 2006), destabilization of target gene mRNAs through the insertion of a selectable marker in the 3'-UTR of essential genes (Schuldiner et al. 2005), systematic addition of a heat-inducible degron to the amino terminus of the protein product (Labib et al. 2000), systematic generation of novel temperature-sensitive alleles (Ben-Aroya et al 2008), and systematic integration of existing temperature- sensitive alleles (Li et al. 2011). Despite the availability of several essential gene collections, no one collection is com- plete, suggesting that complementary approaches using a number of screening strategies and multiple types of con- ditional alleles will be necessary to identify all of the es- sential genes that function to suppress genomic instability Here we describe a series of screens to identify essential genes that function to suppress genome instability, using the collection of tetracycline-regulated promoter replacement alleles (Tet alleles) of essential genes (Mnaimneh et al 2004). We screened 217 Tet alleles of essential genes whose depletion caused accumulation in S or G2 phases of the cell cycle (Yu et al. 2006) and identified 47 with elevated levels of spontaneous DNA damage. A second screen performed with the same Tet alleles identified 92 essential genes that suppress the formation of chromosome rearrangements, whole chromosome deletions, and gene conversions. We quantified the levels of each type of mutation in 15 strains that exhibited both elevated levels of spontaneous DNA damage and chro- mosome rearrangements following the depletion of an essen- tial gene. Mapping of rearrangement breakpoints in seven representative mutants from this set revealed several unique previously described for Rad52-YFP (Lisby et al. 2004; Lisby and Rothstein 2004; Chang et al. 2005). Ddc2 foci were quantified in at least 100 cells for each strain. Ddc2 foci in wild-type cells were analyzed four times and used to calcu- late a standard deviation. Tet allele strains that had Ddc2 foci levels that were at least three standard deviations greater than wild type were scored as positive. illegitimate mating assays Tet allele strains and the R1158 wild-type strain were grown in parallel for 24 hr on YPD solid media either containing or lacking 10 Hg/ml of doxycycline. A standard mating assay was performed with tester strains MCY13 (MATa , legiti- mate mating) and MCY14 (MATa, illegitimate mating) on the same media conditions that the strains were grown. Diploids were isolated by replica plating on minimal media. In the quantitative form of this mating assay, Tet allele strains and R1158 wild-type strain were grown in parallel for 24 hr in YPD liquid media containing or lacking 10 ug/ml doxycycline. Strains were mixed with fivefold excess of MCY13, MCY14, or 1225a (MATa his4 thr4) tester strains and plated on YPD solid media. After 5 hr, cells were col- lected, resuspended in water, and plated on diploid selection media. Independent illegitimate diploids were isolated after the mating of the Tet allele strains with the 1225a tester strain. For each mating experiment, 100 diploids were iso- lated and tested for their ability to grow in the presence or absence of histidine or threonine. This assay was repeated two times. Viability of each strain following growth in doxycycline was confirmed by plating on YPD. Only MCM7 (10 %), NUF2 (30%), and UBC9 (50% ) had <100% viability following growth in doxycycline. rearrangement structures. Sequence features, including Ty ret- rotransposons and DNA replication origins and termination zones, correlated with the rearrangements identified. We pro- pose a central role for DNA replication proteins in suppressing the formation of chromosome breaks that promote chromo- Array comparative genome hybridization Genomic DNA was extracted (Qiagen) from independent illegitimate diploids and wild-type diploids isolated from the mating assay. CGH on a microarray was performed as previously described (Dion and Brown 2009) using S. cerevisiae whole genome tiling microarrays (Affymetrix). Signal in tensities of the experimental and wild-type control sam- ples were normalized and compared using tiling analysis software (Affymetrix). Genomic patterns were mapped and analyzed using the integrated genome browser software (Affymetrix). some rearrangements. Materials and Methods Yeast strains and media Tet allele strains were constructed as described previously (Mnaimneh et al. 2004). The genotype of the wild-type Tet allele strain, R1158, is MATa URA3::CMV-tTA his341 leu240 met1540. Using standard genetic methods, 217 MATa Tet allele strains were engineered to contain YFP-Ddc2 marked with a nourseothricin (Nat) resistance gene. Gen otypes for strains used in this study are listed in Table S6. The essential genes that were studied are listed in Table S1 and Table S2. Standard yeast media and growth conditions were used unless otherwise specified (Sherman 1991). CHEF gel electrophoresis and Southern blot analysis Contour-clamped homogenous electric field (CHEF) gels were used to examine intact chromosomes of illegitimate diploids isolated from the mating assay. CHEF gel analysis was performed as described previously (Desany et al 1998). A 1.2 % agarose gel was run at 8 V/cm using pulse times of 120 sec for 30 hr at 14° in 0.5x TBE buffer. PCR- Fluorescence microscopy Tet allele strains were grown in YPD liquid media at 30°, Samples were divided into two cultures and grown in par allel in the presence and absence of 10 Hg/ml doxycycline for 4 additional hours at 23°. Intracellular localization of Ddc2-YFP was determined by fluorescence microscopy as purified fragments were radio labeled by random priming (Stratagene) and used as hybridization probes for Southern blot analysis. PCR primers designed for probe construction are listed in Table S7. E. Cheng et al 148 DIC Ddc2-YFP DIC Ddc2-YFP wildtype DPB11 NSE1 Figure 1 Depletion of yeast essential genes results in el- evated levels of spontaneous Ddc2 foci formation. (A) A total of 217 Tet alleles that express Ddc2-YFP and display a G2/M or S phase cell cycle arrest phenotype were grown in the presence of doxycycline (10 ug/ml) for 4 hr to inhibit the transcription of each essential gene. Representative DIC and YFP images are shown for the wild-type, DPB11 and NSE1 strains. Ddc2-YFP foci are indicated with white -doxycycline (promoter ON) +doxycycline (promoter OFF) 3 45 DNA replication Response to DNA damage Cell Cycle Unknown roles in genome maintenance arrows. (B) The percentage of cells with Ddc2-YFP foci is plotted for 47 Tet alleles that showed an increase in Ddc2 foci of at least three standard deviations above the aver- observed in wild type. Bars are shaded according to the GO process annotation of each gene of interest 20 10 5 Tet alleles discovery rate (FDR) correction were considered strongly significant. Restriction digestion and sequencing analysis of FS1 and FS2 Genomic DNA was isolated (Qiagen) from wild-type strains R1158 and BY4741 and digested with EcoRI and Xbal (New England Biolabs) using the suggested conditions. Digested fragments were separated on a 1% agarose gel and hybrid- ized with FEN2 and FS2-2 probes for Southern blot anal- ysis (Table S7). 5' and 3' ends of fragile site 1 (FS1) and FS2 were PCR amplified and sequenced. PCR primers used for both amplification and sequencing are listed in Table S8. Results Depletion of essential gene products causes spontaneous DNA damage We used a collection of tetracycline-regulated promoter alleles (Tet alleles) (Mnaimneh et al. 2004; Yu et al. 2006) of essential genes to systematically identify genes that sup- press spontaneous DNA damage. Since elevated levels of spontaneous DNA damage should elicit a checkpoint re- sponse and cause cell cycle delay, we screened the 217 strains that accumulated in S phase or G2 phase of the cell cycle following gene-product depletion by promoter shut off (Yu et al. 2006). Spontaneous DNA damage was mea- by the relocalization of the DNA damage checkpoint Enrichment analyses S. cerevisiae chromosomes were broken into 5-kb bins. For each bin, the presence or absence of breakpoints and geno- mic features was tabulated. Various genomic features (Di Rienzi et al. 2009) and replication termination sites (Fachinetti et al. 2010) from previous datasets were used for analysis. For each feature, the total number of bins with both the feature and a breakpoint was determined. To test for enrichment of breakpoints and each feature, a hypergeometric distribu- tion was assumed. P-values <0.05 were considered as ev- idence of a correlation and P-values <0.05 after a false sured protein Ddc2 from a diffuse nuclear pattern to discrete sub- nuclear foci (Figure 1A) (Melo et al. 2001; Lisby et al. 2004). Following growth of these strains in doxycycline to repress essential gene expression, the fraction of cells with Ddc2 foci was quantified (Supporting Information, Table S1). We Essential Genome Stability Genes 149
et al. 2004; Yu et al. 2006), destabilization of target gene mRNAs through the insertion of a selectable marker in the 3'-UTR of essential genes (Schuldiner et al. 2005), systematic addition of a heat-inducible degron to the amino terminus of the protein product (Labib et al. 2000), systematic generation of novel temperature-sensitive alleles (Ben-Aroya et al 2008), and systematic integration of existing temperature- sensitive alleles (Li et al. 2011). Despite the availability of several essential gene collections, no one collection is com- plete, suggesting that complementary approaches using a number of screening strategies and multiple types of con- ditional alleles will be necessary to identify all of the es- sential genes that function to suppress genomic instability Here we describe a series of screens to identify essential genes that function to suppress genome instability, using the collection of tetracycline-regulated promoter replacement alleles (Tet alleles) of essential genes (Mnaimneh et al 2004). We screened 217 Tet alleles of essential genes whose depletion caused accumulation in S or G2 phases of the cell cycle (Yu et al. 2006) and identified 47 with elevated levels of spontaneous DNA damage. A second screen performed with the same Tet alleles identified 92 essential genes that suppress the formation of chromosome rearrangements, whole chromosome deletions, and gene conversions. We quantified the levels of each type of mutation in 15 strains that exhibited both elevated levels of spontaneous DNA damage and chro- mosome rearrangements following the depletion of an essen- tial gene. Mapping of rearrangement breakpoints in seven representative mutants from this set revealed several unique previously described for Rad52-YFP (Lisby et al. 2004; Lisby and Rothstein 2004; Chang et al. 2005). Ddc2 foci were quantified in at least 100 cells for each strain. Ddc2 foci in wild-type cells were analyzed four times and used to calcu- late a standard deviation. Tet allele strains that had Ddc2 foci levels that were at least three standard deviations greater than wild type were scored as positive. illegitimate mating assays Tet allele strains and the R1158 wild-type strain were grown in parallel for 24 hr on YPD solid media either containing or lacking 10 Hg/ml of doxycycline. A standard mating assay was performed with tester strains MCY13 (MATa , legiti- mate mating) and MCY14 (MATa, illegitimate mating) on the same media conditions that the strains were grown. Diploids were isolated by replica plating on minimal media. In the quantitative form of this mating assay, Tet allele strains and R1158 wild-type strain were grown in parallel for 24 hr in YPD liquid media containing or lacking 10 ug/ml doxycycline. Strains were mixed with fivefold excess of MCY13, MCY14, or 1225a (MATa his4 thr4) tester strains and plated on YPD solid media. After 5 hr, cells were col- lected, resuspended in water, and plated on diploid selection media. Independent illegitimate diploids were isolated after the mating of the Tet allele strains with the 1225a tester strain. For each mating experiment, 100 diploids were iso- lated and tested for their ability to grow in the presence or absence of histidine or threonine. This assay was repeated two times. Viability of each strain following growth in doxycycline was confirmed by plating on YPD. Only MCM7 (10 %), NUF2 (30%), and UBC9 (50% ) had <100% viability following growth in doxycycline. rearrangement structures. Sequence features, including Ty ret- rotransposons and DNA replication origins and termination zones, correlated with the rearrangements identified. We pro- pose a central role for DNA replication proteins in suppressing the formation of chromosome breaks that promote chromo- Array comparative genome hybridization Genomic DNA was extracted (Qiagen) from independent illegitimate diploids and wild-type diploids isolated from the mating assay. CGH on a microarray was performed as previously described (Dion and Brown 2009) using S. cerevisiae whole genome tiling microarrays (Affymetrix). Signal in tensities of the experimental and wild-type control sam- ples were normalized and compared using tiling analysis software (Affymetrix). Genomic patterns were mapped and analyzed using the integrated genome browser software (Affymetrix). some rearrangements. Materials and Methods Yeast strains and media Tet allele strains were constructed as described previously (Mnaimneh et al. 2004). The genotype of the wild-type Tet allele strain, R1158, is MATa URA3::CMV-tTA his341 leu240 met1540. Using standard genetic methods, 217 MATa Tet allele strains were engineered to contain YFP-Ddc2 marked with a nourseothricin (Nat) resistance gene. Gen otypes for strains used in this study are listed in Table S6. The essential genes that were studied are listed in Table S1 and Table S2. Standard yeast media and growth conditions were used unless otherwise specified (Sherman 1991). CHEF gel electrophoresis and Southern blot analysis Contour-clamped homogenous electric field (CHEF) gels were used to examine intact chromosomes of illegitimate diploids isolated from the mating assay. CHEF gel analysis was performed as described previously (Desany et al 1998). A 1.2 % agarose gel was run at 8 V/cm using pulse times of 120 sec for 30 hr at 14° in 0.5x TBE buffer. PCR- Fluorescence microscopy Tet allele strains were grown in YPD liquid media at 30°, Samples were divided into two cultures and grown in par allel in the presence and absence of 10 Hg/ml doxycycline for 4 additional hours at 23°. Intracellular localization of Ddc2-YFP was determined by fluorescence microscopy as purified fragments were radio labeled by random priming (Stratagene) and used as hybridization probes for Southern blot analysis. PCR primers designed for probe construction are listed in Table S7. E. Cheng et al 148 DIC Ddc2-YFP DIC Ddc2-YFP wildtype DPB11 NSE1 Figure 1 Depletion of yeast essential genes results in el- evated levels of spontaneous Ddc2 foci formation. (A) A total of 217 Tet alleles that express Ddc2-YFP and display a G2/M or S phase cell cycle arrest phenotype were grown in the presence of doxycycline (10 ug/ml) for 4 hr to inhibit the transcription of each essential gene. Representative DIC and YFP images are shown for the wild-type, DPB11 and NSE1 strains. Ddc2-YFP foci are indicated with white -doxycycline (promoter ON) +doxycycline (promoter OFF) 3 45 DNA replication Response to DNA damage Cell Cycle Unknown roles in genome maintenance arrows. (B) The percentage of cells with Ddc2-YFP foci is plotted for 47 Tet alleles that showed an increase in Ddc2 foci of at least three standard deviations above the aver- observed in wild type. Bars are shaded according to the GO process annotation of each gene of interest 20 10 5 Tet alleles discovery rate (FDR) correction were considered strongly significant. Restriction digestion and sequencing analysis of FS1 and FS2 Genomic DNA was isolated (Qiagen) from wild-type strains R1158 and BY4741 and digested with EcoRI and Xbal (New England Biolabs) using the suggested conditions. Digested fragments were separated on a 1% agarose gel and hybrid- ized with FEN2 and FS2-2 probes for Southern blot anal- ysis (Table S7). 5' and 3' ends of fragile site 1 (FS1) and FS2 were PCR amplified and sequenced. PCR primers used for both amplification and sequencing are listed in Table S8. Results Depletion of essential gene products causes spontaneous DNA damage We used a collection of tetracycline-regulated promoter alleles (Tet alleles) (Mnaimneh et al. 2004; Yu et al. 2006) of essential genes to systematically identify genes that sup- press spontaneous DNA damage. Since elevated levels of spontaneous DNA damage should elicit a checkpoint re- sponse and cause cell cycle delay, we screened the 217 strains that accumulated in S phase or G2 phase of the cell cycle following gene-product depletion by promoter shut off (Yu et al. 2006). Spontaneous DNA damage was mea- by the relocalization of the DNA damage checkpoint Enrichment analyses S. cerevisiae chromosomes were broken into 5-kb bins. For each bin, the presence or absence of breakpoints and geno- mic features was tabulated. Various genomic features (Di Rienzi et al. 2009) and replication termination sites (Fachinetti et al. 2010) from previous datasets were used for analysis. For each feature, the total number of bins with both the feature and a breakpoint was determined. To test for enrichment of breakpoints and each feature, a hypergeometric distribu- tion was assumed. P-values <0.05 were considered as ev- idence of a correlation and P-values <0.05 after a false sured protein Ddc2 from a diffuse nuclear pattern to discrete sub- nuclear foci (Figure 1A) (Melo et al. 2001; Lisby et al. 2004). Following growth of these strains in doxycycline to repress essential gene expression, the fraction of cells with Ddc2 foci was quantified (Supporting Information, Table S1). We Essential Genome Stability Genes 149
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:Elaine N. Marieb, Katja N. Hoehn
Chapter1: The Human Body: An Orientation
Section: Chapter Questions
Problem 1RQ: The correct sequence of levels forming the structural hierarchy is A. (a) organ, organ system,...
Related questions
Question
Can you explain a brief description of each of the methods used?

Transcribed Image Text:et al. 2004; Yu et al. 2006), destabilization of target gene
mRNAs through the insertion of a selectable marker in the
3'-UTR of essential genes (Schuldiner et al. 2005), systematic
addition of a heat-inducible degron to the amino terminus of
the protein product (Labib et al. 2000), systematic generation
of novel temperature-sensitive alleles (Ben-Aroya et al
2008), and systematic integration of existing temperature-
sensitive alleles (Li et al. 2011). Despite the availability of
several essential gene collections, no one collection is com-
plete, suggesting that complementary approaches using
a number of screening strategies and multiple types of con-
ditional alleles will be necessary to identify all of the es-
sential genes that function to suppress genomic instability
Here we describe a series of screens to identify essential
genes that function to suppress genome instability, using the
collection of tetracycline-regulated promoter replacement
alleles (Tet alleles) of essential genes (Mnaimneh et al
2004). We screened 217 Tet alleles of essential genes whose
depletion caused accumulation in S or G2 phases of the cell
cycle (Yu et al. 2006) and identified 47 with elevated levels
of spontaneous DNA damage. A second screen performed
with the same Tet alleles identified 92 essential genes that
suppress the formation of chromosome rearrangements, whole
chromosome deletions, and gene conversions. We quantified
the levels of each type of mutation in 15 strains that exhibited
both elevated levels of spontaneous DNA damage and chro-
mosome rearrangements following the depletion of an essen-
tial gene. Mapping of rearrangement breakpoints in seven
representative mutants from this set revealed several unique
previously described for Rad52-YFP (Lisby et al. 2004; Lisby
and Rothstein 2004; Chang et al. 2005). Ddc2 foci were
quantified in at least 100 cells for each strain. Ddc2 foci in
wild-type cells were analyzed four times and used to calcu-
late a standard deviation. Tet allele strains that had Ddc2
foci levels that were at least three standard deviations greater
than wild type were scored as positive.
illegitimate mating assays
Tet allele strains and the R1158 wild-type strain were grown
in parallel for 24 hr on YPD solid media either containing or
lacking 10 Hg/ml of doxycycline. A standard mating assay
was performed with tester strains MCY13 (MATa , legiti-
mate mating) and MCY14 (MATa, illegitimate mating) on
the same media conditions that the strains were grown.
Diploids were isolated by replica plating on minimal media.
In the quantitative form of this mating assay, Tet allele
strains and R1158 wild-type strain were grown in parallel
for 24 hr in YPD liquid media containing or lacking 10 ug/ml
doxycycline. Strains were mixed with fivefold excess of
MCY13, MCY14, or 1225a (MATa his4 thr4) tester strains
and plated on YPD solid media. After 5 hr, cells were col-
lected, resuspended in water, and plated on diploid selection
media. Independent illegitimate diploids were isolated after
the mating of the Tet allele strains with the 1225a tester
strain. For each mating experiment, 100 diploids were iso-
lated and tested for their ability to grow in the presence or
absence of histidine or threonine. This assay was repeated two
times. Viability of each strain following growth in doxycycline
was confirmed by plating on YPD. Only MCM7 (10 %), NUF2
(30%), and UBC9 (50% ) had <100% viability following
growth in doxycycline.
rearrangement structures. Sequence features, including Ty ret-
rotransposons and DNA replication origins and termination
zones, correlated with the rearrangements identified. We pro-
pose a central role for DNA replication proteins in suppressing
the formation of chromosome breaks that promote chromo-
Array comparative genome hybridization
Genomic DNA was extracted (Qiagen) from independent
illegitimate diploids and wild-type diploids isolated from the
mating assay. CGH on a microarray was performed as
previously described (Dion and Brown 2009) using S. cerevisiae
whole genome tiling microarrays (Affymetrix). Signal in
tensities of the experimental and wild-type control sam-
ples were normalized and compared using tiling analysis
software (Affymetrix). Genomic patterns were mapped and
analyzed using the integrated genome browser software
(Affymetrix).
some rearrangements.
Materials and Methods
Yeast strains and media
Tet allele strains were constructed as described previously
(Mnaimneh et al. 2004). The genotype of the wild-type
Tet allele strain, R1158, is MATa URA3::CMV-tTA his341
leu240 met1540. Using standard genetic methods, 217 MATa
Tet allele strains were engineered to contain YFP-Ddc2
marked with a nourseothricin (Nat) resistance gene. Gen
otypes for strains used in this study are listed in Table S6.
The essential genes that were studied are listed in Table S1
and Table S2. Standard yeast media and growth conditions
were used unless otherwise specified (Sherman 1991).
CHEF gel electrophoresis and Southern blot analysis
Contour-clamped homogenous electric field (CHEF) gels
were used to examine intact chromosomes of illegitimate
diploids isolated from the mating assay. CHEF gel analysis
was performed as described previously (Desany et al
1998). A 1.2 % agarose gel was run at 8 V/cm using pulse
times of 120 sec for 30 hr at 14° in 0.5x TBE buffer. PCR-
Fluorescence microscopy
Tet allele strains were grown in YPD liquid media at 30°,
Samples were divided into two cultures and grown in par
allel in the presence and absence of 10 Hg/ml doxycycline
for 4 additional hours at 23°. Intracellular localization of
Ddc2-YFP was determined by fluorescence microscopy as
purified fragments were radio labeled by random priming
(Stratagene) and used as hybridization probes for Southern
blot analysis. PCR primers designed for probe construction
are listed in Table S7.
E. Cheng et al
148
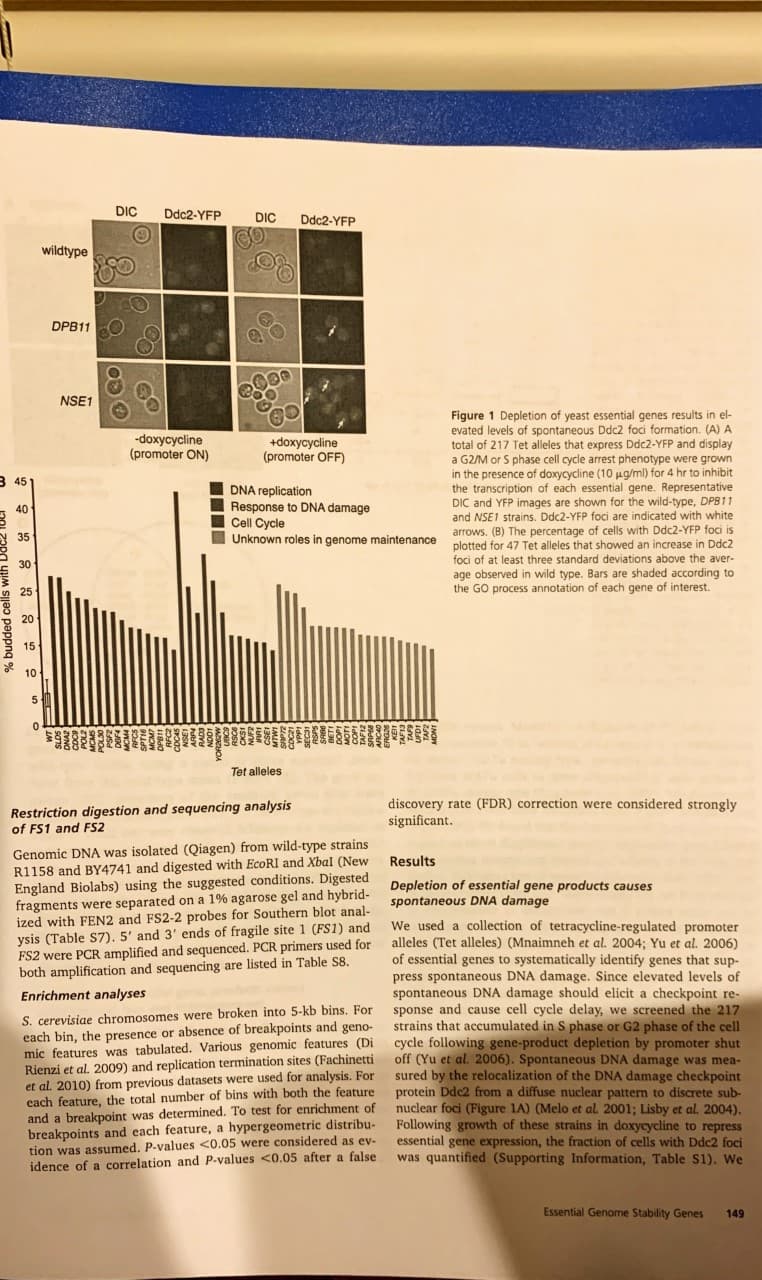
Transcribed Image Text:DIC
Ddc2-YFP
DIC
Ddc2-YFP
wildtype
DPB11
NSE1
Figure 1 Depletion of yeast essential genes results in el-
evated levels of spontaneous Ddc2 foci formation. (A) A
total of 217 Tet alleles that express Ddc2-YFP and display
a G2/M or S phase cell cycle arrest phenotype were grown
in the presence of doxycycline (10 ug/ml) for 4 hr to inhibit
the transcription of each essential gene. Representative
DIC and YFP images are shown for the wild-type, DPB11
and NSE1 strains. Ddc2-YFP foci are indicated with white
-doxycycline
(promoter ON)
+doxycycline
(promoter OFF)
3 45
DNA replication
Response to DNA damage
Cell Cycle
Unknown roles in genome maintenance
arrows. (B) The percentage of cells with Ddc2-YFP foci is
plotted for 47 Tet alleles that showed an increase in Ddc2
foci of at least three standard deviations above the aver-
observed in wild type. Bars are shaded according to
the GO process annotation of each gene of interest
20
10
5
Tet alleles
discovery rate (FDR) correction were considered strongly
significant.
Restriction digestion and sequencing analysis
of FS1 and FS2
Genomic DNA was isolated (Qiagen) from wild-type strains
R1158 and BY4741 and digested with EcoRI and Xbal (New
England Biolabs) using the suggested conditions. Digested
fragments were separated on a 1% agarose gel and hybrid-
ized with FEN2 and FS2-2 probes for Southern blot anal-
ysis (Table S7). 5' and 3' ends of fragile site 1 (FS1) and
FS2 were PCR amplified and sequenced. PCR primers used for
both amplification and sequencing are listed in Table S8.
Results
Depletion of essential gene products causes
spontaneous DNA damage
We used a collection of tetracycline-regulated promoter
alleles (Tet alleles) (Mnaimneh et al. 2004; Yu et al. 2006)
of essential genes to systematically identify genes that sup-
press spontaneous DNA damage. Since elevated levels of
spontaneous DNA damage should elicit a checkpoint re-
sponse and cause cell cycle delay, we screened the 217
strains that accumulated in S phase or G2 phase of the cell
cycle following gene-product depletion by promoter shut
off (Yu et al. 2006). Spontaneous DNA damage was mea-
by the relocalization of the DNA damage checkpoint
Enrichment analyses
S. cerevisiae chromosomes were broken into 5-kb bins. For
each bin, the presence or absence of breakpoints and geno-
mic features was tabulated. Various genomic features (Di
Rienzi et al. 2009) and replication termination sites (Fachinetti
et al. 2010) from previous datasets were used for analysis. For
each feature, the total number of bins with both the feature
and a breakpoint was determined. To test for enrichment of
breakpoints and each feature, a hypergeometric distribu-
tion was assumed. P-values <0.05 were considered as ev-
idence of a correlation and P-values <0.05 after a false
sured
protein Ddc2 from a diffuse nuclear pattern to discrete sub-
nuclear foci (Figure 1A) (Melo et al. 2001; Lisby et al. 2004).
Following growth of these strains in doxycycline to repress
essential gene expression, the fraction of cells with Ddc2 foci
was quantified (Supporting Information, Table S1). We
Essential Genome Stability Genes
149
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:
9780134580999
Author:
Elaine N. Marieb, Katja N. Hoehn
Publisher:
PEARSON

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Anatomy & Physiology
Biology
ISBN:
9781259398629
Author:
McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:
Mcgraw Hill Education,

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:
9780134580999
Author:
Elaine N. Marieb, Katja N. Hoehn
Publisher:
PEARSON

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Anatomy & Physiology
Biology
ISBN:
9781259398629
Author:
McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:
Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:
9780815344322
Author:
Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:
W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:
9781260159363
Author:
Martin, Terry R., Prentice-craver, Cynthia
Publisher:
McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:
9781260231700
Author:
Sylvia S. Mader, Michael Windelspecht
Publisher:
McGraw Hill Education