Ethyl butyrate, CH3 CH₂ CH₂ CO₂ CH₂ CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): ▼ ▼ Part A Given 7.95 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield? Express your answer in grams to three significant figures. ► View Available Hint(s) mass of ethyl butyrate = 10.5 g Submit Previous Answers CH3CH₂CH₂CO₂H(1) + CH₂ CH3OH(1) H, CH3CH₂CH₂CO₂ CH₂ CH3 (1) + H₂O(1) All attempts used; correct answer displayed The mass product produced assuming a 100% yield s called the theoretical yield. Part B A chemist ran the reaction and obtained 5.35 g of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures.
Ethyl butyrate, CH3 CH₂ CH₂ CO₂ CH₂ CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): ▼ ▼ Part A Given 7.95 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield? Express your answer in grams to three significant figures. ► View Available Hint(s) mass of ethyl butyrate = 10.5 g Submit Previous Answers CH3CH₂CH₂CO₂H(1) + CH₂ CH3OH(1) H, CH3CH₂CH₂CO₂ CH₂ CH3 (1) + H₂O(1) All attempts used; correct answer displayed The mass product produced assuming a 100% yield s called the theoretical yield. Part B A chemist ran the reaction and obtained 5.35 g of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 68AP
Related questions
Question
How do i calculate the percent yield?
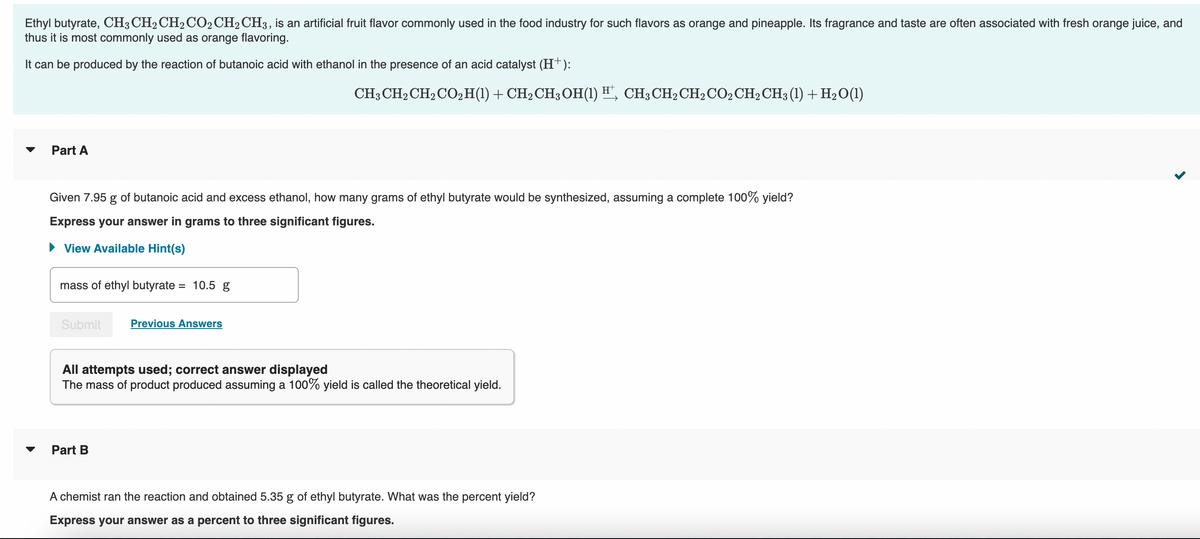
Transcribed Image Text:Ethyl butyrate, CH3 CH2 CH₂ CO2 CH₂ CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and
thus it is most commonly used as orange flavoring.
It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+):
Part A
Given 7.95 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield?
Express your answer in grams to three significant figures.
► View Available Hint(s)
mass of ethyl butyrate = 10.5 g
Submit Previous Answers
CH3 CH₂ CH₂ CO₂ H(1) + CH₂ CH3OH(1) H¹, CH³CH₂CH₂ CO2 CH₂ CH3 (1) + H₂O(1)
All attempts used; correct answer displayed
The mass of product produced assuming a 100% yield is called the theoretical yield.
Part B
A chemist ran the reaction and obtained 5.35 g of ethyl butyrate. What was the percent yield?
Express your answer as a percent to three significant figures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning