etic energy is the energy of motion The following two experiments were designed to explore nether speed or mass plays a bigger role in detemining the amount of kinetic energy. Speriment was designed to test how speed aflects kinelic energy The kinetic energy is alculated using a mass of 1.200 kg (mass of an average car) traveling at various speeds The equation for calculating the kinetic energy of an object is KE-(m. KE the kinetic energy of the object It is measured in the energy unit of joules (J). m the mass of the object in kitograms (kg). Vthe speed of the object, measured in meters per second (m/s), which must be squared. Note 1 joule 1 kg x (m/s) Complete the table for Experiment 1 and find the kinetic energy of the car at differentpeeds. in this experiment, mass is constant, which means that mass does not change. Experiment 1: Kinetic Energy of a 1,200 kg Car at Difforent Speeds Mass of Car Speed of Car Kinetic Energy 1,200 kg 2.78 m/s 1. 1,200 kg 5.55 m/s 2. 3. 1,200 kg 8.33 m/s 1,200 kg 11.11 m/s 1,200 kg 13.89 m/s 6. 1,200 kg 16.67 m/s 7. 1,200 kg 19.44 m/s 4. 5,
etic energy is the energy of motion The following two experiments were designed to explore nether speed or mass plays a bigger role in detemining the amount of kinetic energy. Speriment was designed to test how speed aflects kinelic energy The kinetic energy is alculated using a mass of 1.200 kg (mass of an average car) traveling at various speeds The equation for calculating the kinetic energy of an object is KE-(m. KE the kinetic energy of the object It is measured in the energy unit of joules (J). m the mass of the object in kitograms (kg). Vthe speed of the object, measured in meters per second (m/s), which must be squared. Note 1 joule 1 kg x (m/s) Complete the table for Experiment 1 and find the kinetic energy of the car at differentpeeds. in this experiment, mass is constant, which means that mass does not change. Experiment 1: Kinetic Energy of a 1,200 kg Car at Difforent Speeds Mass of Car Speed of Car Kinetic Energy 1,200 kg 2.78 m/s 1. 1,200 kg 5.55 m/s 2. 3. 1,200 kg 8.33 m/s 1,200 kg 11.11 m/s 1,200 kg 13.89 m/s 6. 1,200 kg 16.67 m/s 7. 1,200 kg 19.44 m/s 4. 5,
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 38QAP: A gasoline station in Manila, Philippines, charges 38.46 pesos per liter of unleaded gasoline at a...
Related questions
Question
100%
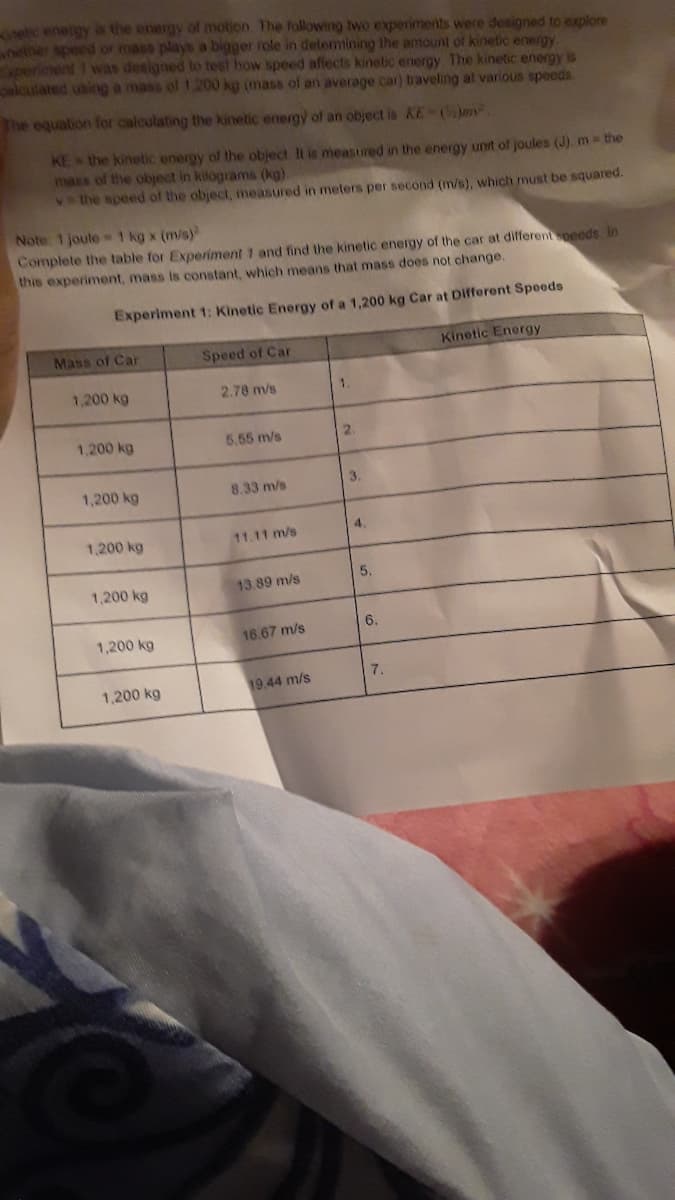
Transcribed Image Text:etic energy is the energy of motion. The following two experiments were designed to explore
nether speed or mass plays a bigger role in delemining the amount of kinetic energy.
Speriment 1 was designed to test how speed afects kinetic energy The kinetic energy is
alcutated using a mass of 1.200 kg (mass of an average car) traveling at various speeds
he equation for calculating the kinetic energy of an object is KE-Cm
KE the kinetic energy of the object It is measured in the energy unit of joules (J). m the
mass of the object in klograms (kg)
Vthe speed of the object, measured in meters per second (m/s), which must be squared.
Note 1 joule1 kg x (m/s)
Complete the table for Experiment 1 and find the kinetic energy of the car at differentpeeds in
this experiment, mass is constant, which means that mass does not change.
Experiment 1: Kinetic Energy of a 1,200 kg Car at Difforent Speeds
Mass of Car
Speed of Car
Kinetic Energy
1,200 kg
2.78 m/s
1
1,200 kg
5.55 m/s
2.
3.
1,200 kg
8.33 m/s
4
1,200 kg
11.11 m/s
5.
1,200 kg
13.89 m/s
6.
1,200 kg
16.67 m/s
7.
1,200 kg
19.44 m/s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning