The heat equation, as given in the introduction, can also be rearranged to calculate the mass or temperature change for a substance. Follow the same steps used to calculate the quantity of heat gained or lost, but when you solve the equation, the term for mass or temperature change must be isolated on one side of the equation. What mass, in grams, of aluminum fins could 2298 J of energy heat from 13.61 °C to 23.64 °C? Aluminum has a specific heat of 0.897 (J/g) · °C. Express your answer to three significant figures. » View Available Hint(s) Mass =
The heat equation, as given in the introduction, can also be rearranged to calculate the mass or temperature change for a substance. Follow the same steps used to calculate the quantity of heat gained or lost, but when you solve the equation, the term for mass or temperature change must be isolated on one side of the equation. What mass, in grams, of aluminum fins could 2298 J of energy heat from 13.61 °C to 23.64 °C? Aluminum has a specific heat of 0.897 (J/g) · °C. Express your answer to three significant figures. » View Available Hint(s) Mass =
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 15P
Related questions
Question
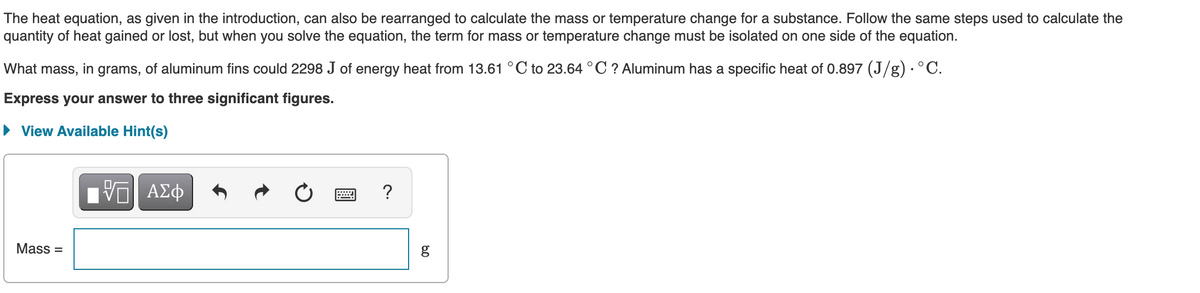
Transcribed Image Text:The heat equation, as given in the introduction, can also be rearranged to calculate the mass or temperature change for a substance. Follow the same steps used to calculate the
quantity of heat gained or lost, but when you solve the equation, the term for mass or temperature change must be isolated on one side of the equation.
What mass, in grams, of aluminum fins could 2298 J of energy heat from 13.61 °C to 23.64 °C ? Aluminum has a specific heat of 0.897 (J/g) · ° C.
Express your answer to three significant figures.
• View Available Hint(s)
Ηνα ΑΣφ
?
Mass =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning