EX/ A sample of argon (molar mass 39.9 gmol) of mass 6.56 g occupies 18.5 dm' at 305 K. (a) Calculate the work done when the gas expands isothermally against a constant external pressure of 7.7 kPa until its volume has increased by 2.5 dm. (b) Calculate the work that would be done if the same expansion occurred reversibly.
EX/ A sample of argon (molar mass 39.9 gmol) of mass 6.56 g occupies 18.5 dm' at 305 K. (a) Calculate the work done when the gas expands isothermally against a constant external pressure of 7.7 kPa until its volume has increased by 2.5 dm. (b) Calculate the work that would be done if the same expansion occurred reversibly.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter17: Chemcial Thermodynamics
Section: Chapter Questions
Problem 17.103QE: A 220-ft3 sample of gas at standard temperature and pressure is compressed into a cylinder, where it...
Related questions
Question
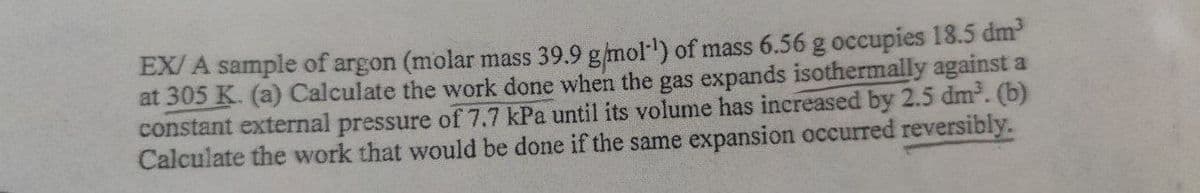
Transcribed Image Text:EX/ A sample of argon (molar mass 39.9 g/mol') of mass 6.56 g occupies 18.5 dm
at 305 K. (a) Calculate the work done when the gas expands isothermally against a
constant external pressure of 7.7 kPa until its volume has increased by 2.5 dm. (b)
Calculate the work that would be done if the same expansion occurred reversibly.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER