Exactly 0.2220 g of pure NazCO; was dissolved in 100.0 mL of 0.0731 M HCI. What mass in grams of CO2 were evolved? Na2CO3 + 2HCI → 2NaCl + CO2 + H2O Na=23 C=12 0=16 CI=35.5 H=1
Q: 23A 0.20 g sample of primary standard Na2C204 (134 g/mol) needed 37.22 mL of of KMNO4 solution to…
A:
Q: A (3.6500 g) of impure ammonium aluminium sulfate (NH4AI(SO4)2)) was treated with ammonia (NH3(aq))…
A: Given: Mass of sample = 3.6500 g. Mass of Al2O3 formed = 0.4935 g.
Q: Nitrite (NO2) can be determined by oxidation with excess Ce4*, followed by back titration of the…
A: CHEM 108b labChemical Reactivity in the MarineEnvironmentProfessor Rachel Narehood…
Q: An unknown 8.00 ml sample containing a certain concentration of tin Sn*2 (118.7 g/mol), was treated…
A:
Q: Addition of 50.00 mL of 2.238 m H2SO4 (solution density = 1.1243 g/mL) to 50.00 mL of 2.238 M BaCl2…
A:
Q: dissolved and diluted to 300 mL with water. A 20 mL aliquot was A 15.00 g sample containing mixed…
A:
Q: An impure sample of KCIO, (FW = 122.5 g/mol) weighing 0.1353 grams was dissolved in 50.0 mL…
A: By considering balanced reaction for individual steps we can figure out mass of KClO3 present in the…
Q: How many grams of BaF2 (molar mass = 175.337) will dissolve in 600 mL of 0.45 M NaF solution? The…
A: Solubility of any salt is defined as the maximum amount of solute that can be dissolve in a solvent…
Q: Mass of KxFe(C2O4)y · zH2O : 4.70 g Mass of sample : 0.175 g Mass of FeCl3 used in preparation :…
A: Mass of KxFe(C2O4)y · zH2O : 4.70 g Mass of sample : 0.175 g Mass of FeCl3 used in preparation :…
Q: A 5.00 gram sample containing NaCl was treated with 50 mL of 0.1995N AgNO3 and required 10.45 mL of…
A: Here we are required to find percentage purity of NaCl.
Q: A 0.64 g sample containing KCl ( mw = 74.6 ) is dissolved in 50mL of water and titrated to the…
A: The %W/W of KCl in the solution has to be given,
Q: mixture containing only KCl and NaBr is analyzed by the Mohr Method. A 0.3172-g sample is dissolved…
A:
Q: An impure sample of Na3PO3 weighing 0.1 g is dissolved in 35 mL of water. A solution containing 45…
A: The final sample does not contain Na3PO3 as the precipitate contains only Hg2Cl2. The question will…
Q: 1. A 1.000 g sample.containing Na,C,0, (MM=134 mg/mmol) is titrated with 40.00 mL of 0.0200 M KMNO,…
A: Given,
Q: 1. A student synthesized 3.214g of Ni(NH3),Cl2 from 4.023g of NiCl2 6H₂O. He dissolved 0.81g of…
A: To find out the concentration of Ni2+ and the mass percent of nickel in the product.
Q: Step 1 :4.9668 g of Na2SO3 was dissolved in 30ml distilled water by heating. Step 2 : Then 6.0895g…
A: In a reaction, the interaction between the two reactants result in the formation of product. The…
Q: A 2.950 sample containing NH,CI, Rb,CO3 and inert material was dissolved to give 250.0 ml of…
A: Therefore,
Q: An analyte solution was prepared using 1.1278 g of a diprotic acid and 25.0 mL of distilled water. A…
A:
Q: A 0.951-g sample containing (NH4)2C2O4 and compounds was dissolved in water and made alkaline with…
A:
Q: A 0.4435-g sample of impure Na2CO3 (FW 105.99) was analyzed by the Volhard method. After adding…
A:
Q: A 25.00 cm3 solution containing Ni2+ was treated with an excess of an ammoniacal solution of…
A:
Q: The thiourea in a 1.455-g sample of organic material was extracted into a dilute H2SO4 solution and…
A: The given reaction is as follows: 4NH22CS + Hg2+ →NH22CS4Hg2+ Calculation of number of moles of…
Q: A 0.4755-g sample containing (NH4)2C2O4 and inert compounds was dissolved in water and made alkaline…
A: Interpretation: The percentage of N and (NH4)2C2O4 in the sample are to be determined. Given: Sample…
Q: * OWLV2 | Online teachinX C The Deep Blue Compound (+…
A: Concentration of the solution (solution 2) obtained on diluting 0.164 M HCl can be determined as…
Q: The digestion of a 0.1159 gram sample of a phosphorous-containing compound in a mixture of HNO3 and…
A:
Q: Zhongli is a speleologist tasked to analyze the CaCO₃ content of a limestone stalactite. A 5.0000-g…
A: An acid is a substance that can furnish hydrogen ions in an aqueous solution. Besides the strong…
Q: The digestion of a 0 1432 g sample of a compound containing phosphorous in a mixture of HNO3 and…
A: Given: Mass of sample = 0.1432 g. Volume of NaOH added = 50.00 mL = 0.050 L…
Q: A (3.6500 g) of impure ammonium aluminium sulfate (NH4Al(SO4)2)) was treated with ammonia (NH3(aq))…
A: Given: Mass of sample = 3.6500 g. Mass of Al2O3 formed = 0.4935 g.
Q: A rock was analyzed for its copper content. Since copper goes through redox reactions, an iodometric…
A: To determine the copper content in a rock sample.
Q: A 0.1093-g sample of impure Na2CO3( Molecular weight 106) was analyzed by the Volhard method. After…
A: Given: 1) The reaction taking place: Na2CO3 + 2AgNO3 → Ag2CO3 + 2NaNO3 2) The reaction of KSCN with…
Q: S,0g? (aq) + C20,?(aq) → 2SO,2 (ae) + 2CO2(g) ter reaction with 50.00 mL of 0.05006 M Na2C204, the…
A: Molarity of sodium oxalate = 0.05006 M Volume of sodium oxalate = 50.0 mL
Q: The CO in a 20.3-L sample of gas was converted to CO2 by passing the gas over iodine pentoxide…
A: Given reactions are: I2O5(s) + 5CO (g) → 5CO2(g) + I2 (g) I2(aq) + 2S2O32- (aq) → 2I-(aq) + S4O62-…
Q: An aqueous ethylene glycol (HOCH₂CH₂OH, FW = 62.07 g/mol) solution with a mass of 214.3 mg is…
A:
Q: A 1.219-g containing (NH4)2SO4, NH4NO3, and nonreactive substances was diluted to 200 mL in a…
A: Given: For the first titration, Mass of the given sample = 1.219g Volume of the sample = 200 mL…
Q: Exactly 0.1120g of pure Na2CO3 was dissolved in 100.0 ml of 0.0497 M HClO 4 . What mass in grams of…
A: The amount of CO2 can be determined from the limiting reagent. The limiting reagent of a reaction is…
Q: The ethyl acetate concentration in a alcoholic solution was determined by diluting a 10.00 mL sample…
A:
Q: Mass of KxFe(C2O4)y · zH2O : 5.60 g Mass of sample : 0.155 g Mass of FeCl3 used in preparation :…
A: The given compound first undergoes dissociation in its aqueous solution as:
Q: A student synthesized 3.214g of Ni(NH3),Cl2 from 4.023g of NiCl2•6H2O. He dissolved 0.81g of…
A:
Q: A sample known to consist of NaOH orNaHCO3, or Na2CO3 or possible compatible mixtures of these,…
A: 2Na2CO3+2HCL⇌2NaHCO3+2NaCL b) 2NaHCO3+2HCL⇌2NaCL+2H2CO3
Q: Ascorbic acid (Vitamin C, MW = 176.126g/mol) is a reducing agent, reacting as follows: C6H8O6 →…
A: Given data, Volume of sample = 200mL = 0.2L Molarity of I2 = 0.05 M Volume of I2 = 10mL = 0.01L…
Q: The formaldehyde content of a pesticide preparation was determined by weighing 0.3124 g of the…
A: The balanced chemical reaction is as follows: HCHO + OH- + H2O2 = HCOO- + 2 H2O The no of mmol =…
Q: Mass of KxFe(C2O4)y · zH2O : 5.60 g Mass of sample : 0.155 g Mass of FeCl3 used in preparation :…
A: The given compound undergoes dissociation in its aqueous solution as :
Q: 3. A 4.326 g sample containing ethyl mercaptan (C₂H_SH) was treated with 50.00 mL of 0.1287 M 1₂ in…
A: The number of moles of a substance is given as the mass of the substance upon its molar mass. The…
Q: The gravimetric determination of N²": Ni (58.7 g/mol) was precipitated as Ni(DMG) ; (288.7 g/mol).…
A: A multiple choice question based on gravimetric analysis, which is to be accomplished.
Q: how many grams of BaF2 molar mass= 175.337 will dissolve in 300 mL of 0.30 M NaF solution? the ksp…
A:
Q: Mass of KxFe(C2O4)y · zH2O : 5.60 g Mass of sample : 0.155 g Mass of FeCl3 used in preparation :…
A: The valency of Potassium is known ( which is equal to 1), but he valency of the othercounterpart is…
Q: A 2.036 g of copper (II) salt was dissolved in a 250 mL volumetric flask. A 25 mL aliquot of the…
A:
Q: A 0.951-g sample containing (NH4)2C2O4 and compounds was dissolved in water and made alkaline with…
A: The law of chemical equivalence states that the number of reacting equivalents of all the reactants…
Q: The Zn in a 0.8872-g sample of foot powder was titrated with 24.24 mL of 0.01842 M EDTA. Calculate…
A: Given data: The volume of EDTA=24.24 mL=0.02424 L. The molarity of EDTA=0.01842 M = 0.01842 mol/L.…
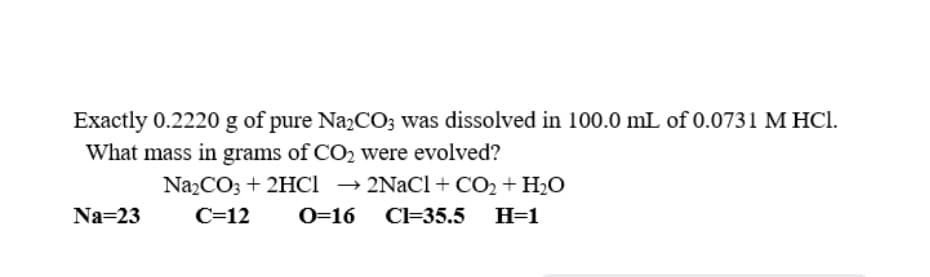
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- Exactly 0.1120g of pure Na2CO3 was dissolved in 100.0 ml of 0.0497 M HClO 4 . What mass in grams of CO 2 was evolved?A mixture containing only KCl and NaBr is analyzed by the Mohr Method. A 0.3172-g sample is dissolved in 50 mL water and titrated to the Ag2CrO4 endpoint, requiring 36.85 mL of 0.1120 M AgNO3. A blank titration requires 0.71 mL of titrant to reach the same endpoint. Report the % (w/w) KCl and NaBr in the sample. [Answer should be 82.41 % (w /w)]A 0.1093-g sample of impure Na2CO3 was analyzed by the Volhard Method. After adding 50.00 mL of 0.06911 M AgNO3, the sample was back titrated with 0.05781 M KSCN, requiring 27.36 mL to reach the end point. Report the purity of Na2CO3sample.
- A sample weighing 0.6760 g that contains an unknown amount of BaCl₂ was completely dissolved in water and treated with an excess of sodium sulfate. Na2SO4 A precipitate of BaSO4 formed which was dried and weighed, yielding 0.4105 g. What percentage of the original sample was BaCl₂? 39.77 24.15 35.72 69.78 54.180.500 g of an impure ammonium sulfate sample is dissolved in water and treated with an excess of NaOH; The mixture is distilled, and the distillate is taken up in 50.00 mL of 0.2000 M HCl. The solution obtained is titrated with 19.70 mL of 0.2000 M NaOH. Calculate the purity (%) of the ammonium sulfate (MW, 132.14) in the original sample.A 0.1093-g sample of impure Na2CO3 was analyzed by the Volhard Method. After adding 50.00 mL of 0.06911 M AgNO3, the sample was back titrated with 0.05781 M KSCN, requiring 27.36 mL to reach the end point. Report the purity of Na2CO3 sample.
- Percentage purity of a sample of 0.1350 g of As2O3 assayed iodometrically using 23.5 mL of 0.1055N iodine solutionA mixture containing only KCl and NaBr is analyzed by the Mohr method. A 0.5072-g sample is dissolved in 50 mL of water and titrated to the Ag2CrO4 end point, requiring 36.85 mL of 0.1120 M AgNO3. A blank titration requires 0.71 mL of titrant to reach the same end point. Report the %w/w KCl and NaBr in the sample.KCl = 74.551 NaBr = 102.89A 0.1093-g sample of impure Na2CO3( Molecular weight 106) was analyzed by the Volhard method. After adding 50.00 mL of 0.06911 M AGN03 (Molecular weight 169.87), the sample was back titrated with 0.05781 M KSCN, requiring 27.36 mL to reach the end point. Report the purity of the Na2CO3 sample.
- An analysis for borohydride ion is based on its reaction with Ag+: BH4- + 8Ag+ + 8OH- → H2BO3- + 8Ag(s) + 5H2O The purity of a quantity of KBH4 to be used in an organic synthesis was established by diluting 3.258g of the material to exactly 500.0mL, treating a 100.0 mL aliquot with 50.00 mL of 0.2639M AgNO3 and titrating the excess silver ion with 3.36 mL of 0.0397 M KSCN. Calculate the percent purity of the KBH4 [Report the numerical value of the result to two decimal places - NO UNITS]A 1.250 g sample of cheese was subjected to a Kjeldahl analysis to determine the amount of protein. The sample was digested, the nitrogen is oxidized to NH₄⁺, and was then converted to NH₃ with NaOH, and distilled into a collection flask containing 50.00 mL of 0.1050 M HCl. The excess HCl is back titrated with 0.1175 M NaOH, this required 21.65 mL to reach the bromothymol blue end point. A. How many moles of N is present in the cheese sample? B. Report the %N of the cheese sample. C. Report the %protein of the cheese sample, assuming that there are 6.4 grams of protein for every gram of nitrogenA 1.250 g sample of cheese was subjected to a Kjeldahl analysis to determine the amount of protein. The sample was digested, the nitrogen is oxidized to NH₄⁺, and was then converted to NH₃ with NaOH, and distilled into a collection flask containing 50.00 mL of 0.1050 M HCl. The excess HCl is back titrated with 0.1175 M NaOH, this required 21.65 mL to reach the bromothymol blue end point. What is the reaction for the digestion of the limestone sample A. 2 NaOH + CaCO₃ ⇌ Na₂CO₃ + Ca(OH)₂ B. 2 HCl + CaCO₃ ⇌ CaCl₂ + H₂O + CO₂ C. CaCO₃ ⇌ CaO + CO₂ D. NaOH + HCl ⇌ NaCl + H₂O

