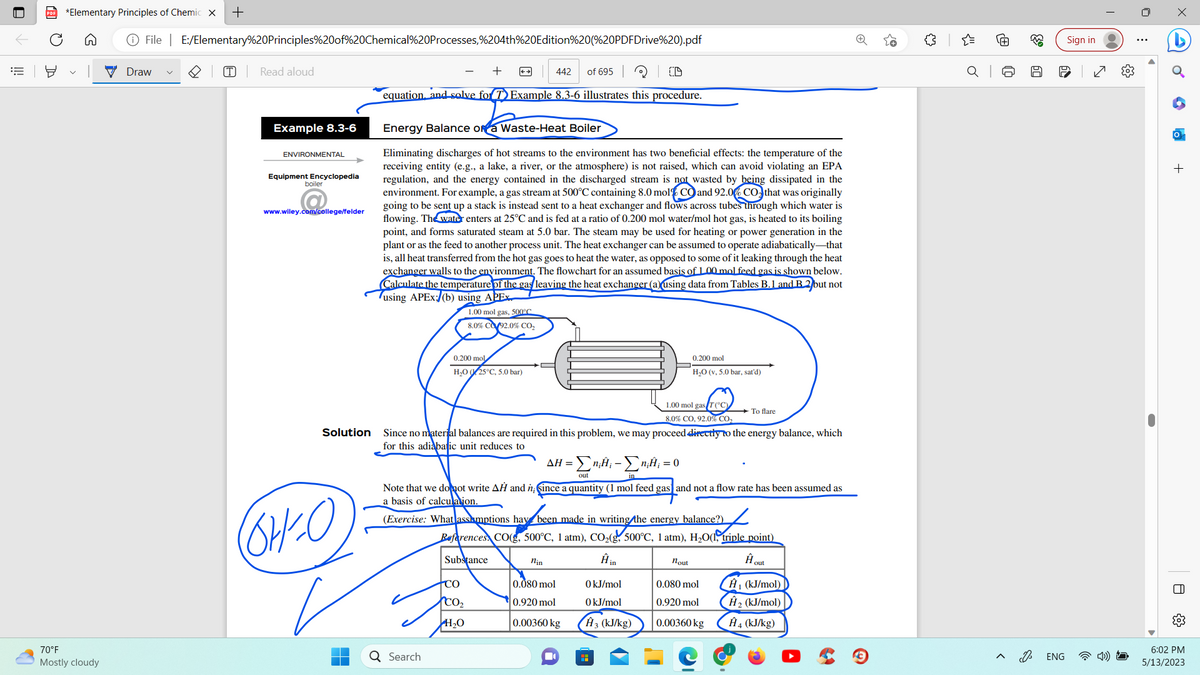
Example 8.3-6 ENVIRONMENTAL Equipment Encyclopedia www.wiley.com/college/felder Energy Balance ora Waste-Heat Boiler Eliminating discharges of hot streams to the environment has two beneficial effects: the temperature of the receiving entity (e.g., a lake, a river, or the atmosphere) is not raised, which can avoid violating an EPA regulation, and the energy contained in the discharged stream is not wasted by being dissipated in the environment. For example, a gas stream at 500°C containing 8.0 mol CO and 92.06 CO that was originally going to be sent up a stack is instead sent to a heat exchanger and flows across tubes through which water is flowing. The water enters at 25°C and is fed at a ratio of 0.200 mol water/mol hot gas, is heated to its boiling point, and forms saturated steam at 5.0 bar. The steam may be used for heating or power generation in the plant or as the feed to another process unit. The heat exchanger can be assumed to operate adiabatically that is, all heat transferred from the hot gas goes to heat the water, as opposed to some of it leaking through the heat exchanger walls to the environment. The flowchart for an assumed basis of 1.00 mal feed gas is shown below. Calculate the temperature of the gas leaving the heat exchanger (ausing data from Tables B.1 and B2 but not using APEx:/(b) using APEX 1.00 mol gas, 500C 8.0% CO2.0% CO₂ 0.200 mol H₂025°C, 5.0 bar) 100 0.200 mol H₂O (v. 5.0 bar, sard)
Example 8.3-6 ENVIRONMENTAL Equipment Encyclopedia www.wiley.com/college/felder Energy Balance ora Waste-Heat Boiler Eliminating discharges of hot streams to the environment has two beneficial effects: the temperature of the receiving entity (e.g., a lake, a river, or the atmosphere) is not raised, which can avoid violating an EPA regulation, and the energy contained in the discharged stream is not wasted by being dissipated in the environment. For example, a gas stream at 500°C containing 8.0 mol CO and 92.06 CO that was originally going to be sent up a stack is instead sent to a heat exchanger and flows across tubes through which water is flowing. The water enters at 25°C and is fed at a ratio of 0.200 mol water/mol hot gas, is heated to its boiling point, and forms saturated steam at 5.0 bar. The steam may be used for heating or power generation in the plant or as the feed to another process unit. The heat exchanger can be assumed to operate adiabatically that is, all heat transferred from the hot gas goes to heat the water, as opposed to some of it leaking through the heat exchanger walls to the environment. The flowchart for an assumed basis of 1.00 mal feed gas is shown below. Calculate the temperature of the gas leaving the heat exchanger (ausing data from Tables B.1 and B2 but not using APEx:/(b) using APEX 1.00 mol gas, 500C 8.0% CO2.0% CO₂ 0.200 mol H₂025°C, 5.0 bar) 100 0.200 mol H₂O (v. 5.0 bar, sard)
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
How was 0.00360 kg H20 obtained for in and out?
Transcribed Image Text:PDF *Elementary Principles of Chemic X +
70°F
Mostly cloudy
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDFDrive%20).pdf
Draw
T Read aloud
Example 8.3-6
ENVIRONMENTAL
Equipment Encyclopedia
boiler
www.wiley.com/college/felder
Solution
(OH-O
+
442 of 695 O OD
equation and solve for T Example 8.3-6 illustrates this procedure.
Energy Balance of a Waste-Heat Boiler
Eliminating discharges of hot streams to the environment has two beneficial effects: the temperature of the
receiving entity (e.g., a lake, a river, or the atmosphere) is not raised, which can avoid violating an EPA
regulation, and the energy contained in the discharged stream is not wasted by being dissipated in the
environment. For example, a gas stream at 500°C containing 8.0 mol% CO and 92.0% CO that was originally
going to be sent up a stack is instead sent to a heat exchanger and flows across tubes through which water is
flowing. The water enters at 25°C and is fed at a ratio of 0.200 mol water/mol hot gas, is heated to its boiling
point, and forms saturated steam at 5.0 bar. The steam may be used for heating or power generation in the
plant or as the feed to another process unit. The heat exchanger can be assumed to operate adiabatically that
is, all heat transferred from the hot gas goes to heat the water, as opposed to some of it leaking through the heat
exchanger walls to the environment. The flowchart for an assumed basis of 1.00 mol feed gas is shown below.
Calculate the temperature of the gas leaving the heat exchanger (a) using data from Tables B.1 and B 2 but not
using APEx:/(b) using APEX.
0.200 mol
H₂O 25°C, 5.0 bar)
1.00 mol gas, 500°C
8.0% C 92.0% CO,
1.00 mol gas T(°C)
8.0% CO, 92.0% CO₂
Since no material balances are required in this problem, we may proceed directly to the energy balance, which
for this adiabatic unit reduces to
Q Search
out
Note that we do not write AH and n; since a quantity (1 mol feed gas and not a flow rate has been assumed as
a basis of calculation.
(Exercise: What assumptions have been made in writing the energy balance?)
+co
AH = Σn;H₁ - Σn;; = 0
in
CO₂
H₂O
References CO, 500°C, 1 atm), CO₂(g, 500°C, 1 atm), H₂O(1, triple point)
Substance
Ĥ in
Ĥ out
nin
0.200 mol
H₂O (v, 5.0 bar, sat'd)
0.080 mol
0.920 mol
0.00360 kg
0 kJ/mol
0 kJ/mol
A3 (kJ/kg)
H
nout
To flare
0.080 mol
0.920 mol
0.00360 kg
A₁ (kJ/mol)
Ĥ₂ (kJ/mol)
A4 (kJ/kg)
{"
J
63
50
D
ENG
Sign in
(0)
+
0
6:02 PM
5/13/2023
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The