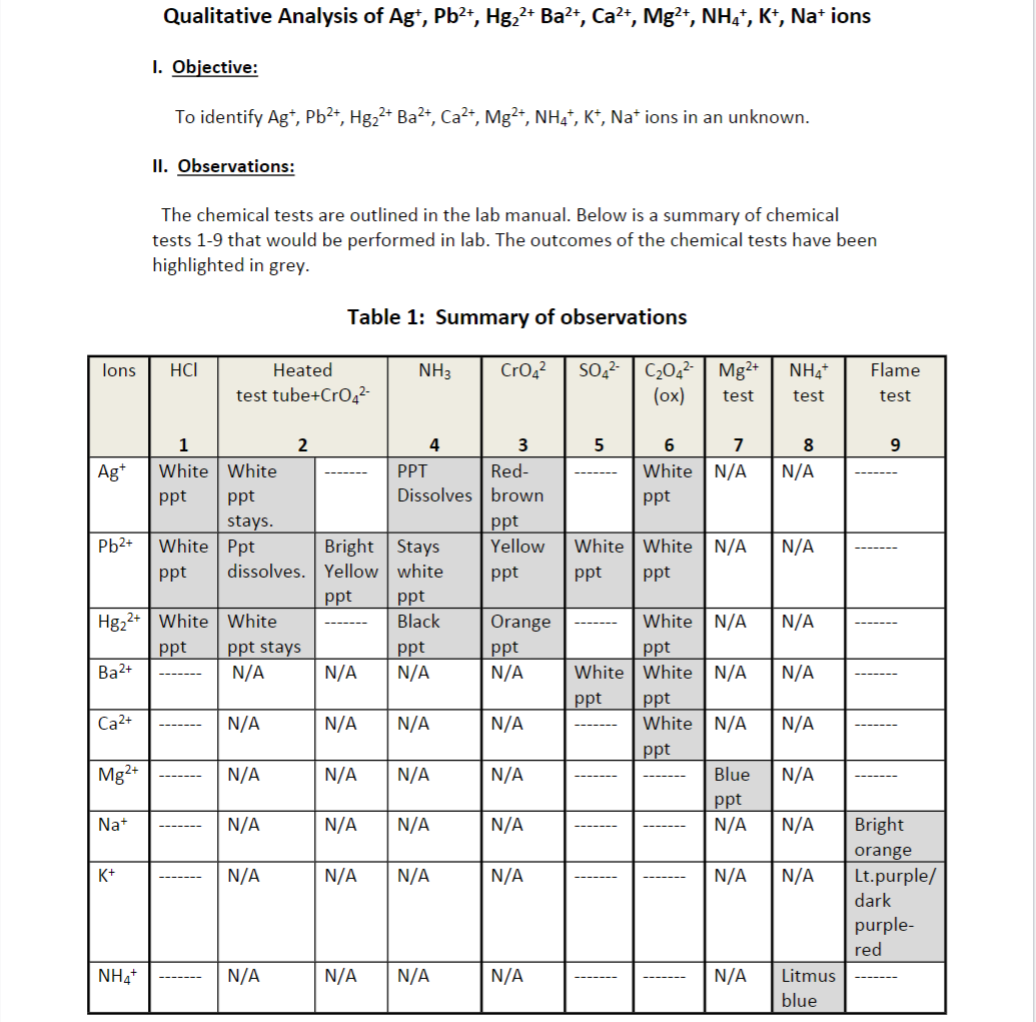
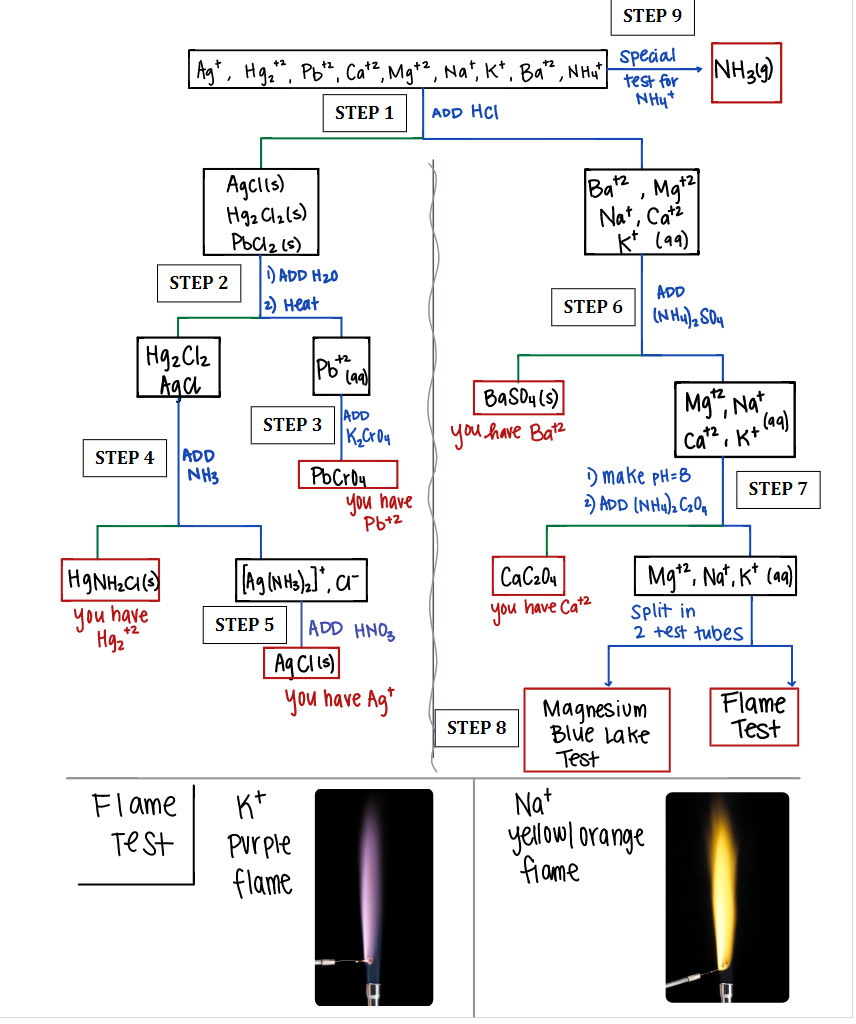
Use the information in the pictures if needed and solve for the unknown D. please type the answers because it is much easier for me to understand that way. Unknown D To the unknown mixture, 3 drops of 6 M HCl was added and no precipitate formed. To the liquid that was left, 3 drops of 0.10M (NH4)2SO4 was added and a precipitate formed. The precipitate was isolated by centrifuging and the liquid from the top decanted for further testing. To the decanted liquid, the solution was made basic and a few drops of (NH4)2C2O4 was added. No precipitate formed. The liquid was tested for Mg2+ and a bright blue jelly ppt formed. To the unknown, a separate NH4+ test was done and the litmus paper used to test the pH turned blue. To the unknown, a flame test was performed and the color emitted was a light purple-red. What ions are in the unknown? (Identify unknown ions and provide a brief summary) DO NOT SIMPLY RESTATE THE ABOVE OBSERVATIONS.
Use the information in the pictures if needed and solve for the unknown D.
please type the answers because it is much easier for me to understand that way.
Unknown D
To the unknown mixture, 3 drops of 6 M HCl was added and no precipitate formed. To the liquid that was left, 3 drops of 0.10M (NH4)2SO4 was added and a precipitate formed. The precipitate was isolated by centrifuging and the liquid from the top decanted for further testing. To the decanted liquid, the solution was made basic and a few drops of (NH4)2C2O4 was added. No precipitate formed. The liquid was tested for Mg2+ and a bright blue jelly ppt formed.
To the unknown, a separate NH4+ test was done and the litmus paper used to test the pH turned blue.
To the unknown, a flame test was performed and the color emitted was a light purple-red. What ions are in the unknown?
(Identify unknown ions and provide a brief summary)
DO NOT SIMPLY RESTATE THE ABOVE OBSERVATIONS.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps




