Exercise 6.8: Make a nested dictionary from a file The viscosity u of gases depends on the temperature. For some gases the following formula is relevant: To -C (T\15 To - C µ(T) = HoT+C (T, where the values of the constants C, To, and Ho are found in the file src/ dictstring/viscosity_of_gases.dat". The temperature is measured in Kelvin. a) Load the file into a nested dictionary mu_data such that we can look up C, To. and lo for a gas with name name by mu_data[name] [X], where X is 'C' for C, 'T_0' for To, and 'mu_0' for µo- b) Make a function mu (T, gas, mu_data) for computing p(T) for a gas with name gas (according to the file) and information about constants C, To, and µo in mu_data. c) Plot u(T) for air, carbon dioxide, and hydrogen with T e [223, 373].
Exercise 6.8: Make a nested dictionary from a file The viscosity u of gases depends on the temperature. For some gases the following formula is relevant: To -C (T\15 To - C µ(T) = HoT+C (T, where the values of the constants C, To, and Ho are found in the file src/ dictstring/viscosity_of_gases.dat". The temperature is measured in Kelvin. a) Load the file into a nested dictionary mu_data such that we can look up C, To. and lo for a gas with name name by mu_data[name] [X], where X is 'C' for C, 'T_0' for To, and 'mu_0' for µo- b) Make a function mu (T, gas, mu_data) for computing p(T) for a gas with name gas (according to the file) and information about constants C, To, and µo in mu_data. c) Plot u(T) for air, carbon dioxide, and hydrogen with T e [223, 373].
C++ for Engineers and Scientists
4th Edition
ISBN:9781133187844
Author:Bronson, Gary J.
Publisher:Bronson, Gary J.
Chapter2: Problem Solving Using C++using
Section2.3: Data Types
Problem 1E: (Practice) Determine data types appropriate for the following data: a. The average of four grades b....
Related questions
Question
The file needed is the second screenshot posted.
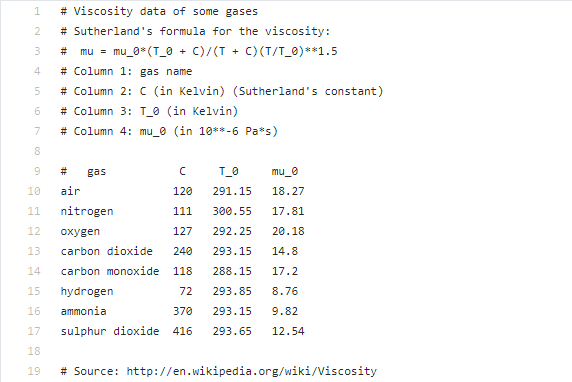
Transcribed Image Text:1
# Viscosity data of some gases
# Sutherland's formula for the viscosity:
mu = mu_0* (T_0 + C)/(T +
C) (T/T_0)**1.5
#
4
# Column 1: gas name
# Column 2: C (in Kelvin) (Sutherland's constant)
# Column 3: T_0 (in Kelvin)
7
# Column 4: mu_0 (in 10**-6 Pa*s)
#3
gas
T_0
mu_0
10
air
120
291.15
18.27
11
nitrogen
111
300.55
17.81
12
охуgen
127
292.25
20.18
13
carbon dioxide
240
293.15
14.8
14
carbon monoxide 118
288.15
17.2
15
hydrogen
72
293.85
8.76
16
ammonia
370
293.15
9.82
17
sulphur dioxide 416
293.65
12.54
18
19
# Source: http://en.wikipedia.org/wiki/Viscosity
ON Co
![Exercise 6.8: Make a nested dictionary from a file
The viscosity u of gases depends on the temperature. For some gases the following
formula is relevant:
To -C (T
μ(T) = HoT + C\To,
1.5
where the values of the constants C, To, and Ho are found in the file src/
dictstring/viscosity_of_gases.dat". The temperature is measured in
Kelvin.
a) Load the file into a nested dictionary mu_data such that we can look up C, To.
and µo for a gas with name name by mu_data[name] [X], where X is 'C' for
C, 'T_0' for To, and 'mu_0'for 4o.
b) Make a function mu(T, gas, mu_data) for computing µ(T) for a gas with
name gas (according to the file) and information about constants C, To, and lo
in mu_data.
c) Plot u(T) for air, carbon dioxide, and hydrogen with T E [223, 373].](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F083bf2fa-560f-4112-a271-9f7ac07ce03b%2Faf3ae122-0070-4191-a613-6977521f9cff%2Fc2ksewrg_processed.png&w=3840&q=75)
Transcribed Image Text:Exercise 6.8: Make a nested dictionary from a file
The viscosity u of gases depends on the temperature. For some gases the following
formula is relevant:
To -C (T
μ(T) = HoT + C\To,
1.5
where the values of the constants C, To, and Ho are found in the file src/
dictstring/viscosity_of_gases.dat". The temperature is measured in
Kelvin.
a) Load the file into a nested dictionary mu_data such that we can look up C, To.
and µo for a gas with name name by mu_data[name] [X], where X is 'C' for
C, 'T_0' for To, and 'mu_0'for 4o.
b) Make a function mu(T, gas, mu_data) for computing µ(T) for a gas with
name gas (according to the file) and information about constants C, To, and lo
in mu_data.
c) Plot u(T) for air, carbon dioxide, and hydrogen with T E [223, 373].
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, computer-science and related others by exploring similar questions and additional content below.Recommended textbooks for you

C++ for Engineers and Scientists
Computer Science
ISBN:
9781133187844
Author:
Bronson, Gary J.
Publisher:
Course Technology Ptr

Systems Architecture
Computer Science
ISBN:
9781305080195
Author:
Stephen D. Burd
Publisher:
Cengage Learning

Enhanced Discovering Computers 2017 (Shelly Cashm…
Computer Science
ISBN:
9781305657458
Author:
Misty E. Vermaat, Susan L. Sebok, Steven M. Freund, Mark Frydenberg, Jennifer T. Campbell
Publisher:
Cengage Learning

C++ for Engineers and Scientists
Computer Science
ISBN:
9781133187844
Author:
Bronson, Gary J.
Publisher:
Course Technology Ptr

Systems Architecture
Computer Science
ISBN:
9781305080195
Author:
Stephen D. Burd
Publisher:
Cengage Learning

Enhanced Discovering Computers 2017 (Shelly Cashm…
Computer Science
ISBN:
9781305657458
Author:
Misty E. Vermaat, Susan L. Sebok, Steven M. Freund, Mark Frydenberg, Jennifer T. Campbell
Publisher:
Cengage Learning

C++ Programming: From Problem Analysis to Program…
Computer Science
ISBN:
9781337102087
Author:
D. S. Malik
Publisher:
Cengage Learning

Programming Logic & Design Comprehensive
Computer Science
ISBN:
9781337669405
Author:
FARRELL
Publisher:
Cengage

Fundamentals of Information Systems
Computer Science
ISBN:
9781305082168
Author:
Ralph Stair, George Reynolds
Publisher:
Cengage Learning