Experiment 1 E Data Table 1 EData Table 2 Exercise 1 Data Table 1: NaOH Titration Volume Initial NaOH Volume (mL) Final NaOH Volume (mL) Total Volume of NaOH Used (mL) Trial 8.9 0.1 8.8 Trial 9.1 0.1 9 Trial 6.9 6.9 Average Volume of NaOH Used (mL):
Experiment 1 E Data Table 1 EData Table 2 Exercise 1 Data Table 1: NaOH Titration Volume Initial NaOH Volume (mL) Final NaOH Volume (mL) Total Volume of NaOH Used (mL) Trial 8.9 0.1 8.8 Trial 9.1 0.1 9 Trial 6.9 6.9 Average Volume of NaOH Used (mL):
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section11.6: Properties Of Liquids
Problem 2.2ACP
Related questions
Question
I need help finding the Average Volume of NaOH Used (mL):
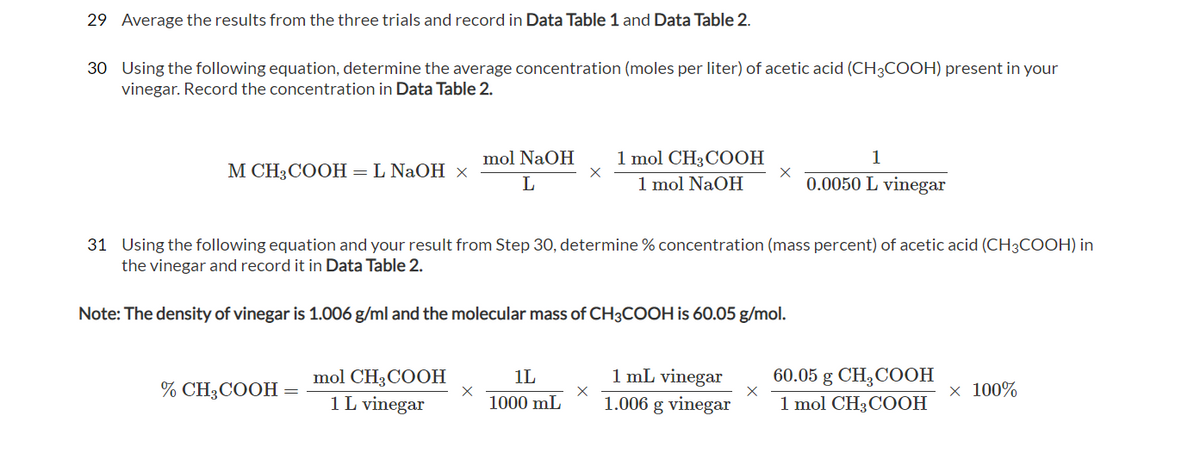
Transcribed Image Text:29 Average the results from the three trials and record in Data Table 1 and Data Table 2.
30 Using the following equation, determine the average concentration (moles per liter) of acetic acid (CH3COOH) present in your
vinegar. Record the concentration in Data Table 2.
1 mol CH3COOH
1 mol NaOH
mol NaOH
1
М СH3СOОН — L NaOH х
0.0050 L vinegar
31 Using the following equation and your result from Step 30, determine % concentration (mass percent) of acetic acid (CH3COOH) in
the vinegar and record it in Data Table 2.
Note: The density of vinegar is 1.006 g/ml and the molecular mass of CH3COOH is 60.05 g/mol.
mol CH;COOН
1L vinegar
1 mL vinegar
1.006 g vinegar
60.05 g CH3COOH
1 mol CH3COOH
1L
% CH3COOH
х 100%
1000 mL
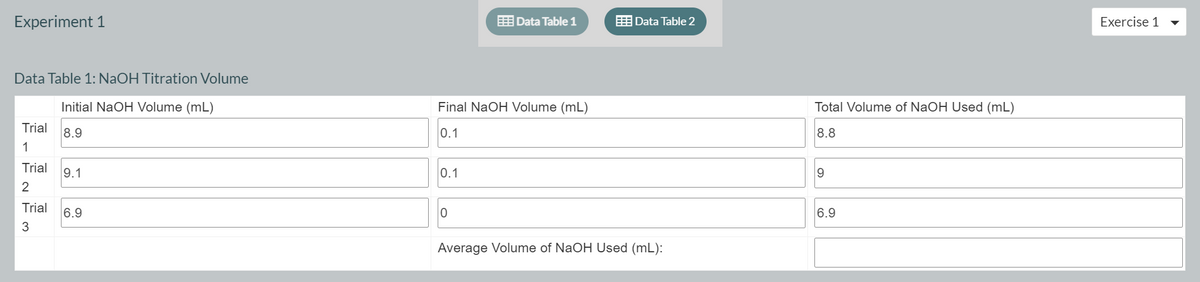
Transcribed Image Text:Experiment 1
E Data Table 1
E Data Table 2
Exercise 1
Data Table 1: NaOH Titration Volume
Initial NaOH Volume (mL)
Final NaOH Volume (mL)
Total Volume of NaOH Used (mL)
Trial
8.9
0.1
8.8
Trial
9.1
0.1
2
Trial
6.9
6.9
3
Average Volume of NaOH Used (mL):
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning