9. 个盟 ANALYSIS PAGE RESULTS TABLE Click the blank box and enter the numbers Volume of H2SO4 = 45 mL Volume of NAOH = mL 2. 3. 7. NaOH Concentration = 0.15 M 4 5 6. H2SO4 Concentration = 0.750 7. SUBMIT C. E: 90 / 120 DAY 1 09:51 PROGRESS: 69% Fill the burette with the correct stand solution, don't forget to use a funnel. THEORY MEDIA MISSION 1. Place the funnel on the burette. 2. Pick the correct standard solution. You can choose between H2SO4, CH3COOH, HCI, and NaOH 3. Pour the standard solution into the burette by clicking the funnel. 4. For now, add minimal 45 mL of titrant into the burette.
9. 个盟 ANALYSIS PAGE RESULTS TABLE Click the blank box and enter the numbers Volume of H2SO4 = 45 mL Volume of NAOH = mL 2. 3. 7. NaOH Concentration = 0.15 M 4 5 6. H2SO4 Concentration = 0.750 7. SUBMIT C. E: 90 / 120 DAY 1 09:51 PROGRESS: 69% Fill the burette with the correct stand solution, don't forget to use a funnel. THEORY MEDIA MISSION 1. Place the funnel on the burette. 2. Pick the correct standard solution. You can choose between H2SO4, CH3COOH, HCI, and NaOH 3. Pour the standard solution into the burette by clicking the funnel. 4. For now, add minimal 45 mL of titrant into the burette.
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 6P
Related questions
Question
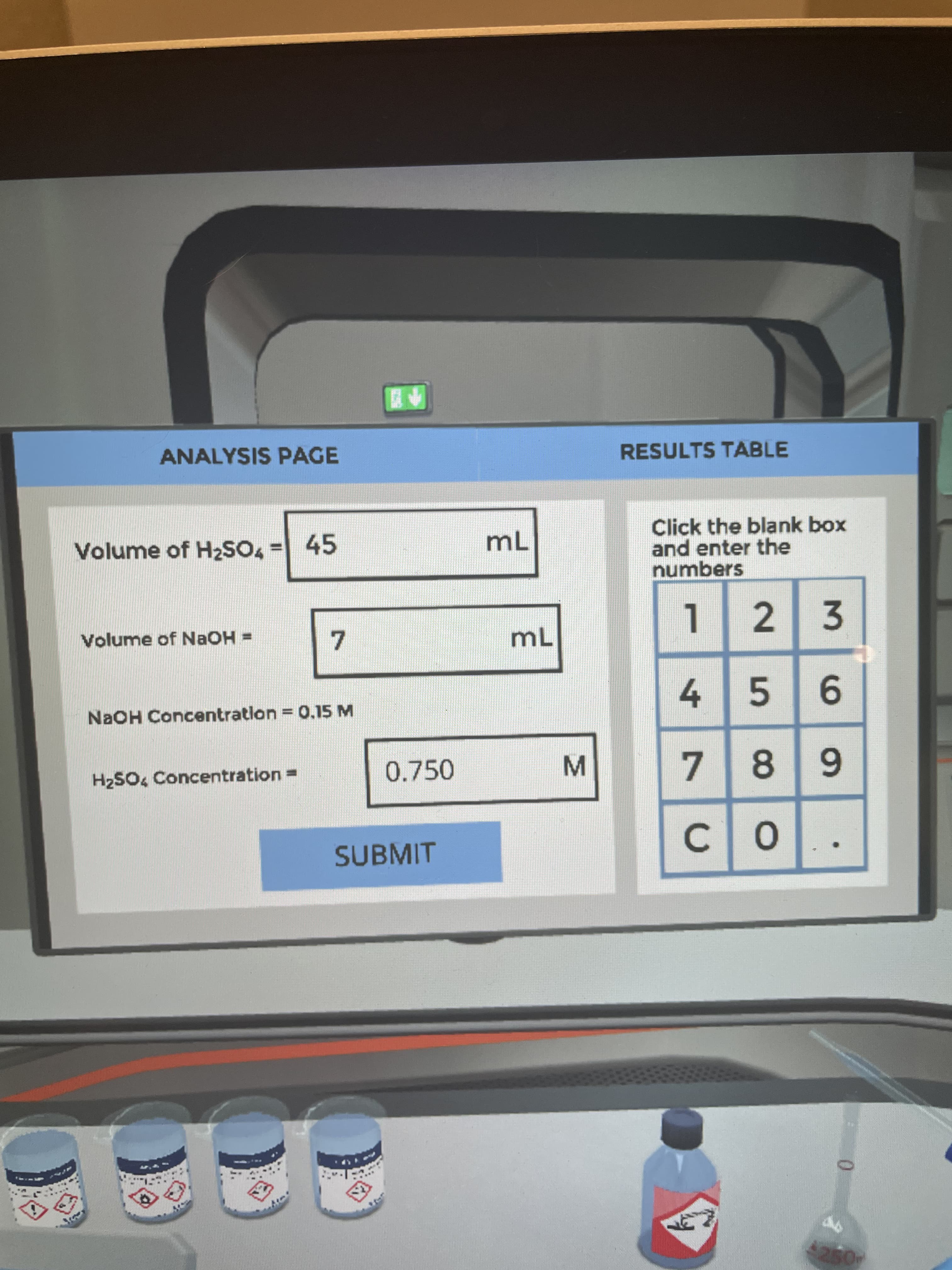
Transcribed Image Text:9.
个盟
ANALYSIS PAGE
RESULTS TABLE
Click the blank box
and enter the
numbers
Volume of H2SO4 = 45
mL
Volume of NAOH =
mL
2.
3.
7.
NaOH Concentration = 0.15 M
4 5
6.
H2SO4 Concentration =
0.750
7.
SUBMIT
C.
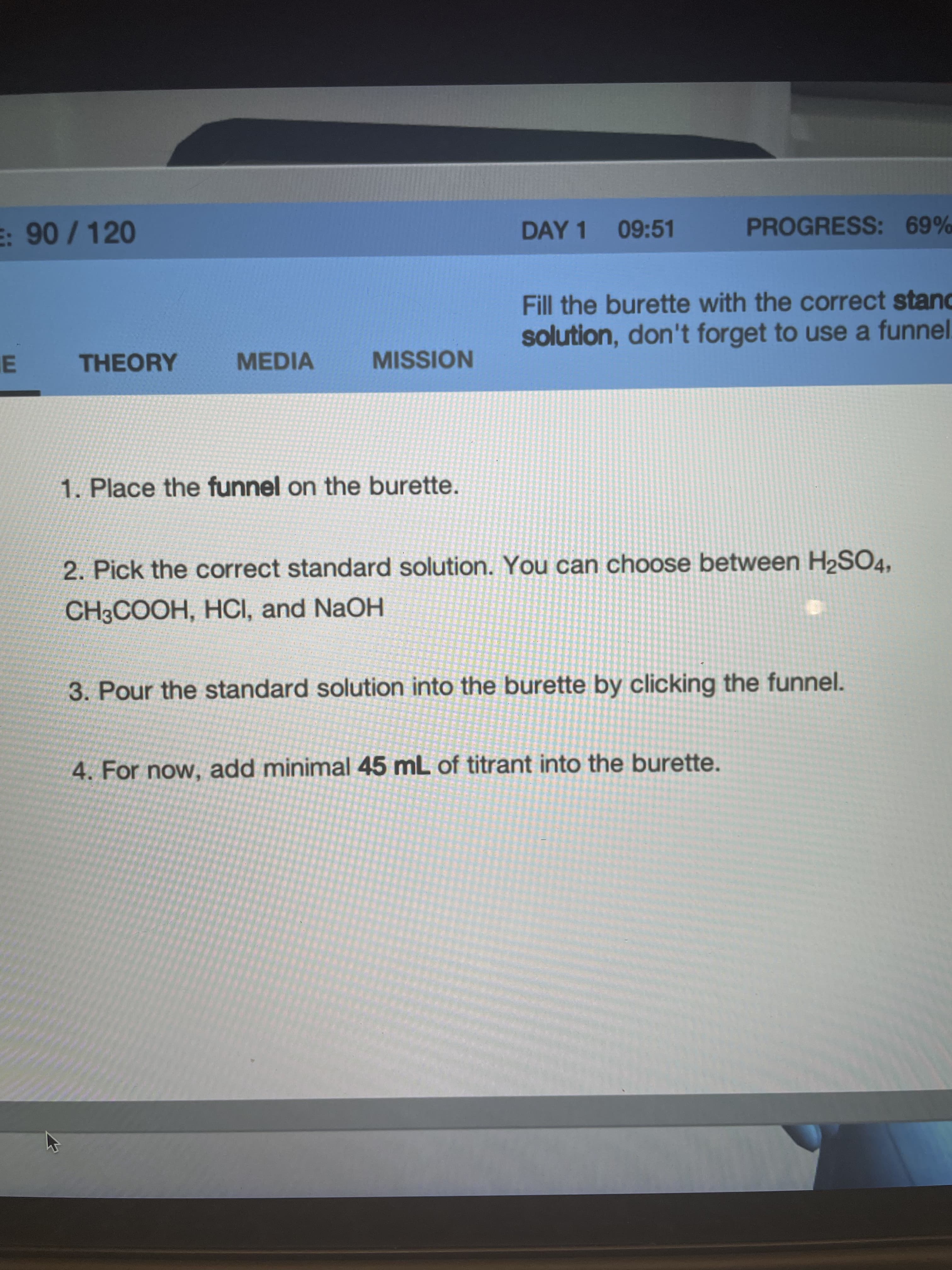
Transcribed Image Text:E: 90 / 120
DAY 1
09:51
PROGRESS: 69%
Fill the burette with the correct stand
solution, don't forget to use a funnel.
THEORY
MEDIA
MISSION
1. Place the funnel on the burette.
2. Pick the correct standard solution. You can choose between H2SO4,
CH3COOH, HCI, and NaOH
3. Pour the standard solution into the burette by clicking the funnel.
4. For now, add minimal 45 mL of titrant into the burette.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning