Explain why each molecule has a higher melting point than the other in the pair. Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. Reset H has a higher molar mass The main reason why Ti(s) has a higher melting point than Ne(s) is that Ti(s) has stronger dipole-dipole interactions The main reason why Fe(s) has a higher melting point than CC (s) is that Fe(s) is an ionic compound can form hydrogen bonds The main reason why KCI(s) has a higher melting point than HCl(s) is that KCI(s) has metallic bonds The main reason why H20(s) has a higher melting point than H2S(s) is that H2O(s)
Explain why each molecule has a higher melting point than the other in the pair. Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. Reset H has a higher molar mass The main reason why Ti(s) has a higher melting point than Ne(s) is that Ti(s) has stronger dipole-dipole interactions The main reason why Fe(s) has a higher melting point than CC (s) is that Fe(s) is an ionic compound can form hydrogen bonds The main reason why KCI(s) has a higher melting point than HCl(s) is that KCI(s) has metallic bonds The main reason why H20(s) has a higher melting point than H2S(s) is that H2O(s)
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter11: States Of Matter; Liquids And Solids
Section: Chapter Questions
Problem 11.93QP
Related questions
Question
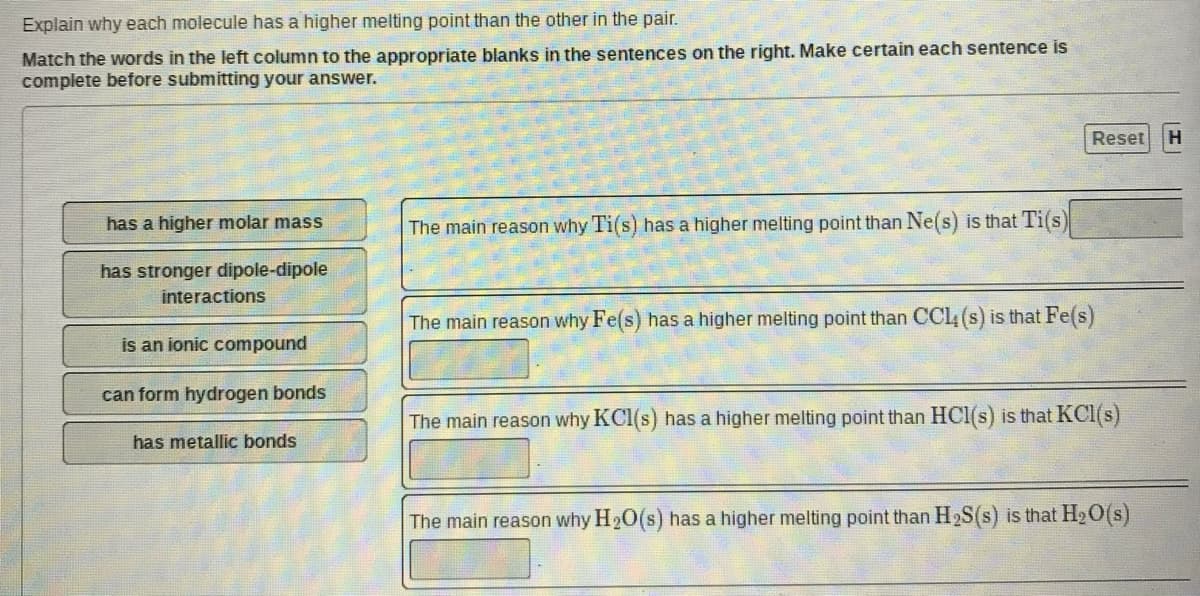
Transcribed Image Text:Explain why each molecule has a higher melting point than the other in the pair.
Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is
complete before submitting your answer.
Reset
has a higher molar mass
The main reason why Ti(s) has a higher melting point than Ne(s) is that Ti(s)
has stronger dipole-dipole
interactions
The main reason why Fe(s) has a higher melting point than CCl (s) is that Fe(s)
is an ionic compound
can form hydrogen bonds
The main reason why KCI(s) has a higher melting point than HCI(s) is that KCI(s)
has metallic bonds
The main reason why H20(s) has a higher melting point than H2S(s) is that H2O(s)
Expert Solution
Step 1
Melting point of any specie is directly proportional to the inter-molecular forces present in the specie.
More is the inter-molecular forces in the specie, more will be the energy required to break those forces and hence more will be the Melting point of the specie.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning