5. Atomic radius, crystal structure, electronegativity, and the most common valence are given in the follo table for several elements; for those that are nonmetals, only atomic radii are indicated. Crystal Structure FCC Element Ni Atomic Radius (nm) 0.1246 Electronegativity 1.8 Valence 0.071 H 0.046 0.060 0.1445 0.1431 0.1253 0.1249 FCC 1.4 1.5 1.7 1.6 +1 Ag Al Co FCC HCP BCC +3 +2 +3 Cr Fe Pt Zn 0.1241 0.1387 ВСС FCC 1.7 1.5 +2 +2 0.1332 HCP 1.7 +2 Which of these elements would you expect to form the following with nickel: (a) A substitutional solid solution having complete solubility (b) A substitutional solid solution of incomplete solubility (c) An interstitial solid solution
5. Atomic radius, crystal structure, electronegativity, and the most common valence are given in the follo table for several elements; for those that are nonmetals, only atomic radii are indicated. Crystal Structure FCC Element Ni Atomic Radius (nm) 0.1246 Electronegativity 1.8 Valence 0.071 H 0.046 0.060 0.1445 0.1431 0.1253 0.1249 FCC 1.4 1.5 1.7 1.6 +1 Ag Al Co FCC HCP BCC +3 +2 +3 Cr Fe Pt Zn 0.1241 0.1387 ВСС FCC 1.7 1.5 +2 +2 0.1332 HCP 1.7 +2 Which of these elements would you expect to form the following with nickel: (a) A substitutional solid solution having complete solubility (b) A substitutional solid solution of incomplete solubility (c) An interstitial solid solution
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter7: Chemical Bonding And Molecular Geometry
Section: Chapter Questions
Problem 75E: Element First Ionization Energy (kJImol) Second Ionization Energy (kiImol) I K 419 3050 Ca 590 1140...
Related questions
Question
100%
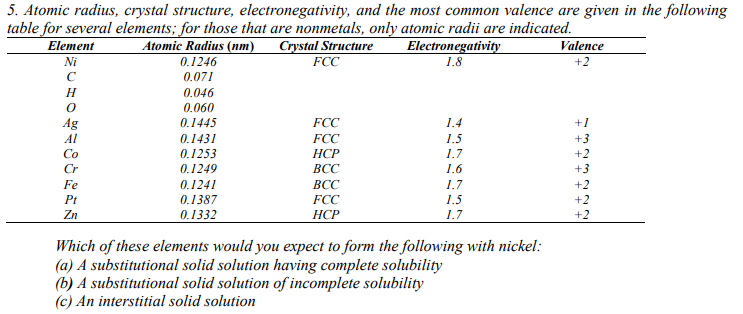
Transcribed Image Text:5. Atomic radius, crystal structure, electronegativity, and the most common valence are given in the follo
table for several elements; for those that are nonmetals, only atomic radii are indicated.
Crystal Structure
FCC
Element
Ni
Atomic Radius (nm)
0.1246
Electronegativity
1.8
Valence
0.071
H
0.046
0.060
0.1445
0.1431
0.1253
0.1249
FCC
1.4
1.5
1.7
1.6
+1
Ag
Al
Co
FCC
HCP
BCC
+3
+2
+3
Cr
Fe
Pt
Zn
0.1241
0.1387
ВСС
FCC
1.7
1.5
+2
+2
0.1332
HCP
1.7
+2
Which of these elements would you expect to form the following with nickel:
(a) A substitutional solid solution having complete solubility
(b) A substitutional solid solution of incomplete solubility
(c) An interstitial solid solution
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning