Chapter4: Organic Compounds: Cycloalkanes And Their Stereochemistry
Section4.3: Stability Of Cycloalkanes: Ring Strain
Problem 8P: Each H↔H eclipsing interaction in ethane costs about 4.0 kJ/mol. How many such interactions are...
Related questions
Question
The table below shows the boiling point points of an
Explain why the solubility of
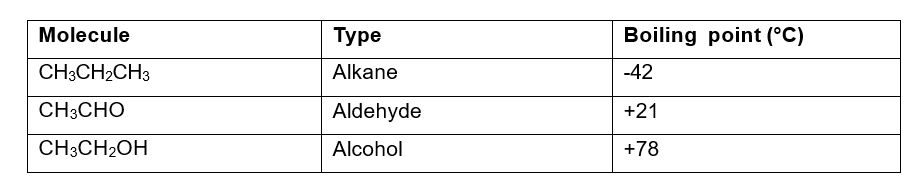
Transcribed Image Text:Molecule
CH3CH₂CH3
CH3CHO
CH3CH₂OH
Type
Alkane
Aldehyde
Alcohol
Boiling point (°C)
-42
+21
+78
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning