Exploration 10D. What combination of simple salts would yield a given composite analysis? Once the concentrations of all significant ions have been established, we are in a position to speculate about ways in which that particular combination of ion concentrations could have originated. For example, a mixture containing Na*, Ca²*, Cl and HCO3 could have come from a mixture of NaCl and Ca(HCO3)2 or from a mixture of NAHCO3 and CaCl2 or from other combinations of these four salts. More than one combination may be possible, but some combinations are ruled out by the data while some combinations are more likelv than others. Exercise 10.4. Propose a mixture of specific salts and determine the mass (in mg) of each of these salts that should be dissolved in one liter of water to yield the concentrations of cations and anions described in Exercises 10.1-10.3. FW g/mol Concentration, ppm Ion Concentration, mM Na+ 1.08 23 24.8 Ca2+ 2.01 40 80.2 Mg2+ 0.96 24 23.3 НСОЗ- 5.594 61 341.7 Cl- 0.46 35.5 16.0 F- 0.0187 19 0.356 NO3- 0.149 62 9.22 SO, 0.399 96 38.3 pH 7.9 Total anions 7.02 TDS calc 533.88 Total cations 7.02 TDS expt 434.3
Exploration 10D. What combination of simple salts would yield a given composite analysis? Once the concentrations of all significant ions have been established, we are in a position to speculate about ways in which that particular combination of ion concentrations could have originated. For example, a mixture containing Na*, Ca²*, Cl and HCO3 could have come from a mixture of NaCl and Ca(HCO3)2 or from a mixture of NAHCO3 and CaCl2 or from other combinations of these four salts. More than one combination may be possible, but some combinations are ruled out by the data while some combinations are more likelv than others. Exercise 10.4. Propose a mixture of specific salts and determine the mass (in mg) of each of these salts that should be dissolved in one liter of water to yield the concentrations of cations and anions described in Exercises 10.1-10.3. FW g/mol Concentration, ppm Ion Concentration, mM Na+ 1.08 23 24.8 Ca2+ 2.01 40 80.2 Mg2+ 0.96 24 23.3 НСОЗ- 5.594 61 341.7 Cl- 0.46 35.5 16.0 F- 0.0187 19 0.356 NO3- 0.149 62 9.22 SO, 0.399 96 38.3 pH 7.9 Total anions 7.02 TDS calc 533.88 Total cations 7.02 TDS expt 434.3
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.1QAP
Related questions
Question
Exercise 10.4

Transcribed Image Text:Exploration 10D. What combination of simple salts would yield a given composite analysis?
Once the concentrations of all significant ions have been established, we are in a position to speculate about
ways in which that particular combination of ion concentrations could have originated. For example, a mixture
containing Na*, Ca²*, Cl and HCO3 could have come from a mixture of NaCl and Ca(HCO3)2 or from a
mixture of NAHCO3 and CaCl2 or from other combinations of these four salts. More than one combination
may be possible, but some combinations are ruled out by the data while some combinations are more likelv
than others.
Exercise 10.4. Propose a mixture of specific salts and determine the mass (in mg) of each of these salts that
should be dissolved in one liter of water to yield the concentrations of cations and anions described in Exercises
10.1-10.3.
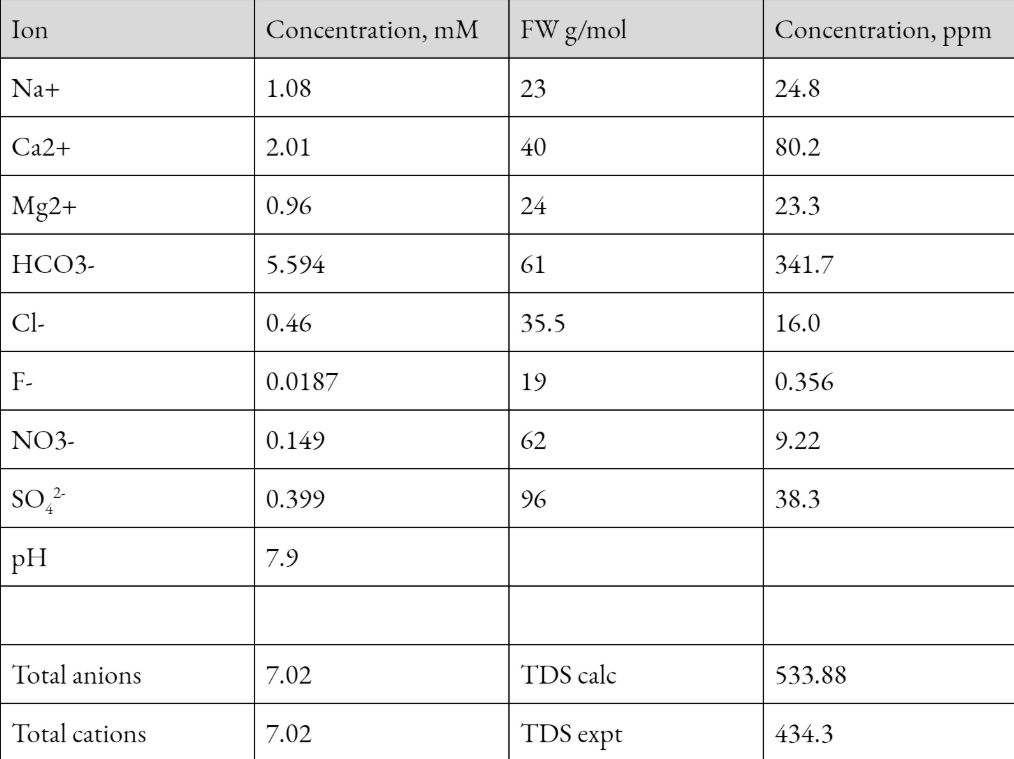
Transcribed Image Text:FW g/mol
Concentration, ppm
Ion
Concentration, mM
Na+
1.08
23
24.8
Ca2+
2.01
40
80.2
Mg2+
0.96
24
23.3
НСОЗ-
5.594
61
341.7
Cl-
0.46
35.5
16.0
F-
0.0187
19
0.356
NO3-
0.149
62
9.22
SO,
0.399
96
38.3
pH
7.9
Total anions
7.02
TDS calc
533.88
Total cations
7.02
TDS expt
434.3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

