Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.58PAE
Related questions
Question
Help me with question 3 plz
Transcribed Image Text:19
BIOLOGY
M.Chapter+19+Second+Lecture+Assignment
V
11.Chapter+19+Second+Lecture+Ass
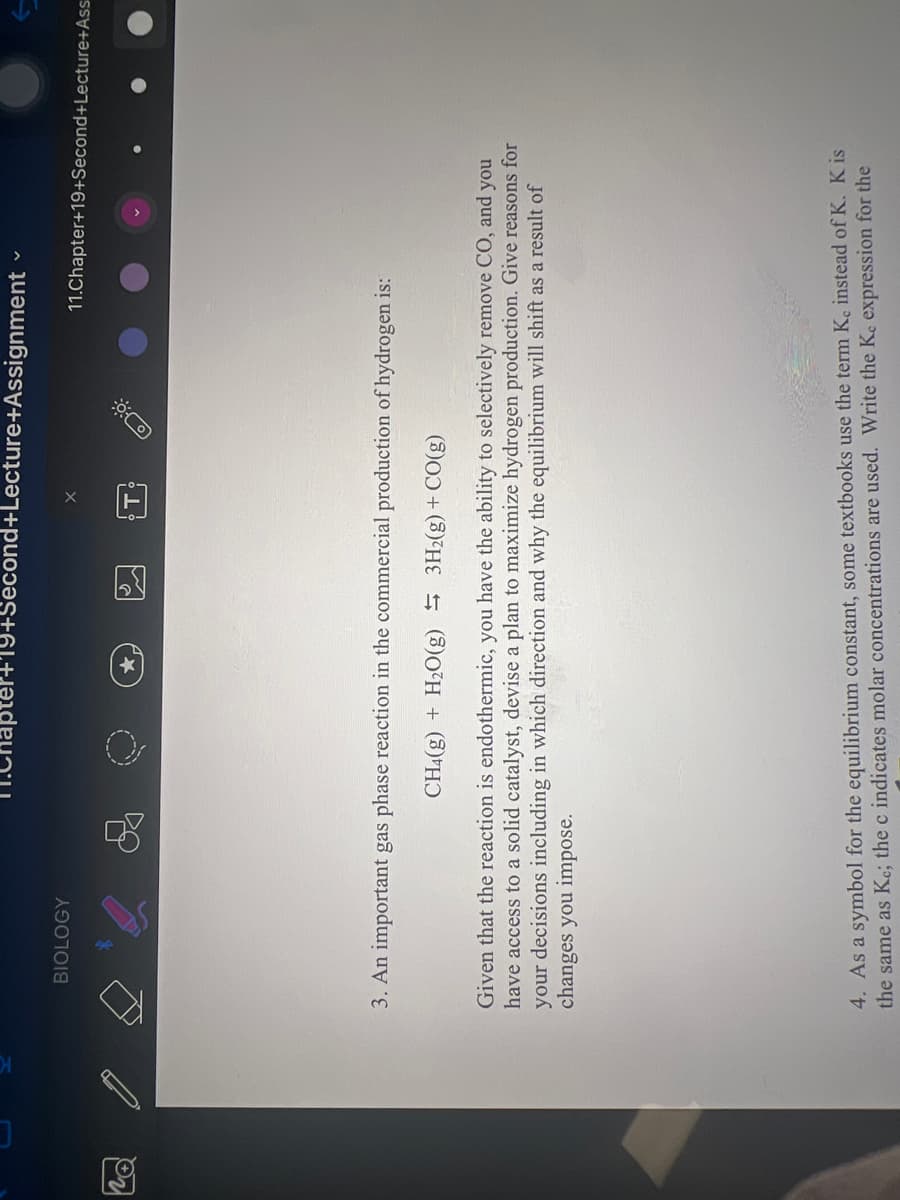
3. An important gas phase reaction in the commercial production of hydrogen is:
CH4(g) + H₂O(g) 3H2(g) + CO(g)
Given that the reaction is endothermic, you have the ability to selectively remove CO, and you
have access to a solid catalyst, devise a plan to maximize hydrogen production. Give reasons for
your decisions including in which direction and why the equilibrium will shift as a result of
changes you impose.
4. As a symbol for the equilibrium constant, some textbooks use the term Ke instead of K. K is
the same as Ke; the c indicates molar concentrations are used. Write the Ke expression for the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
