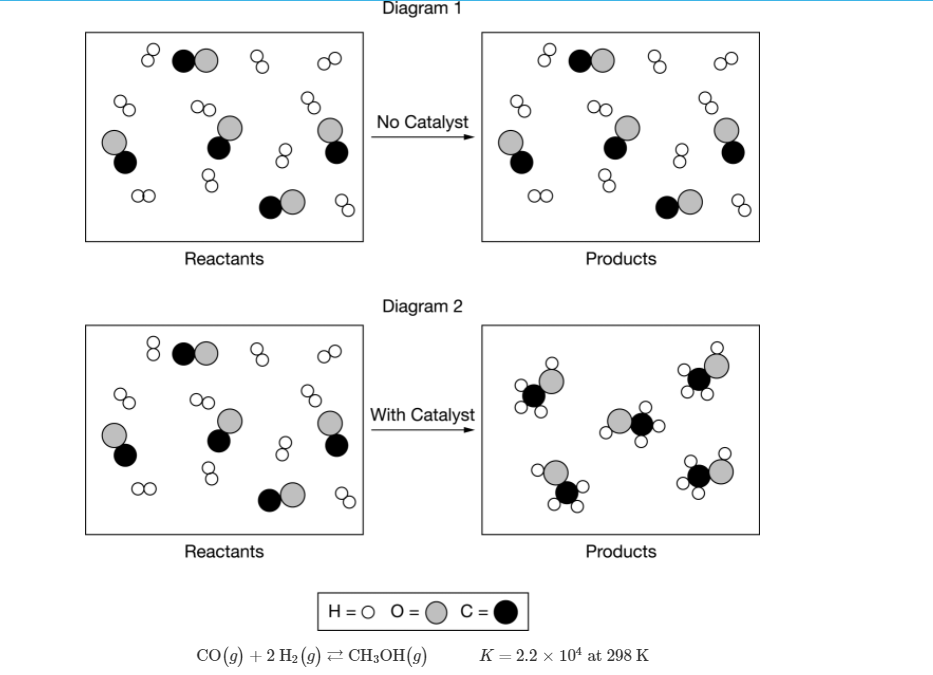
A stoichiometric mixture of CO(g) and H2(g) was allowed to react in two different 2.0L rigid containers at a constant temperature of 298K. The reaction is represented by the equation above. Diagram 1 represents the uncatalyzed reaction and diagram 2 represents the catalyzed reaction one hour after the reactants were mixed. Which of the following correctly explains the experimental results represented in the particle diagrams? A.) Although the reaction is thermodynamically favorable because ΔG°<0 based on the value of K, only the catalyzed reaction could proceed in one hour because its reactant molecules had a higher average kinetic energy. B.) Although the reaction is thermodynamically favorable because ΔG°<0 based on the value of K, only the catalyzed reaction could proceed in one hour because it has a lower activation-energy reaction pathway. C.) The reaction is not thermodynamically favorable because ΔG°>0 based on the value of K, but the addition of a catalyst improved the orientation of the reactants during collisions, allowing the catalyzed reaction to proceed in one hour. D.)The reaction is not thermodynamically favorable because ΔG°>0 based on the value of K, but the catalyzed reaction could proceed in one hour because it has a lower ΔH and a higher ΔS.
A stoichiometric mixture of CO(g) and H2(g) was allowed to react in two different 2.0L rigid containers at a constant temperature of 298K. The reaction is represented by the equation above. Diagram 1 represents the uncatalyzed reaction and diagram 2 represents the catalyzed reaction one hour after the reactants were mixed. Which of the following correctly explains the experimental results represented in the particle diagrams?
A.) Although the reaction is
B.) Although the reaction is thermodynamically favorable because ΔG°<0 based on the value of K, only the catalyzed reaction could proceed in one hour because it has a lower activation-energy reaction pathway.
C.) The reaction is not thermodynamically favorable because ΔG°>0 based on the value of K, but the addition of a catalyst improved the orientation of the reactants during collisions, allowing the catalyzed reaction to proceed in one hour.
D.)The reaction is not thermodynamically favorable because ΔG°>0 based on the value of K, but the catalyzed reaction could proceed in one hour because it has a lower ΔH and a higher ΔS.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









