Fictional, thus not on the periodic table. Write the formula of the compound formed between the transition metal Xx in the +2 oxidation state when reacted with bisulfate.
Fictional, thus not on the periodic table. Write the formula of the compound formed between the transition metal Xx in the +2 oxidation state when reacted with bisulfate.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter19: Transitition Metals, Coordination Chemistry And Metallurgy
Section: Chapter Questions
Problem 19.7QE
Related questions
Question
100%
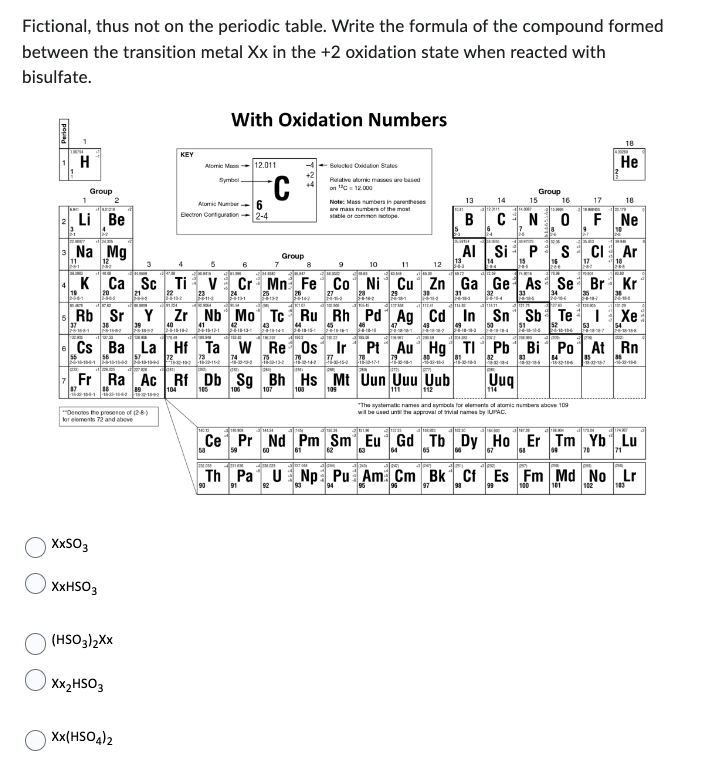
Transcribed Image Text:Fictional, thus not on the periodic table. Write the formula of the compound formed
between the transition metal Xx in the +2 oxidation state when reacted with
bisulfate.
Period
H
Group
87
Li
A
2
Be
3 Na Mg
12
K
Rb Sr Y Zr
XxSO3
Fr Ra Ac
XxHSO3
"Denotes the presence of (2)
for elements 72 and above
3
Sc
(HSO3)2XX
Xx₂HSO3
KEY
Xx(HSO4)2
With Oxidation Numbers
Atomic M12.011
Symbol
Al Number 6
Electron Configuration
2-4
4
6
5
mara
Ti V Cr
20114
C
Rf Db
Group
Zr Nb Mo Tc Ru
bes
Selected Oxidation States
Relative atomic mass and based
on 10 = 12.000
Note: Mass numbers in par
are mass numbers of the most
stable or common isotope
11ܩ ܕܝ
9
THANT
Cs Ba La Hf Ta W Re Os Ir
Pt Au Hg Tl
78
1 PE
Home
house
M P
Sg Bh Hs Mt Uun Uuu Uub
7
10
11
Mn Fe Co Ni Cu Zn Ga
30
5
1000
Rh Pd Ag
MO
514634
Ce Pr Nd Pm Sm
59
20
peme
MCM
MCH
apm
Th Pa U Np Pu
90
91
92
12
48
101
13
13
14
15
16
B C N O
4
Al
TIL
Cd In
14
Si
Uuq
114
Group
E
Eu Gd Tb Dy Ho
63
17
18
F Ne
S Cl Ar
TERTA
As Se Br Kr
Sn Sb Te I Xe
Pb Bi Po
At Rn
2
p
pay
Am Cm Bk Cf Es
93
99
P
"The systematic names and symbols for elements of atomic numbers above 109
will be used until the approval of trivial names by IUPAC.
18
He
Tam
Er Tm Yb Lu
61
Fm Md No Lr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning