Fig. 8 Au 1.0 mol/L KNO, O-1.66 V O +0.16 V +3.16 V of 1.0 mol/L HCI Al(s) 1.0 mol/L AI(NO), The value of the standard cell potential of the cell in Fig. 8 is calculated to be Select one: O +1.66 V
Fig. 8 Au 1.0 mol/L KNO, O-1.66 V O +0.16 V +3.16 V of 1.0 mol/L HCI Al(s) 1.0 mol/L AI(NO), The value of the standard cell potential of the cell in Fig. 8 is calculated to be Select one: O +1.66 V
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 31E: Determine the standard cell potential and the cell potential under the stated conditions for the...
Related questions
Question
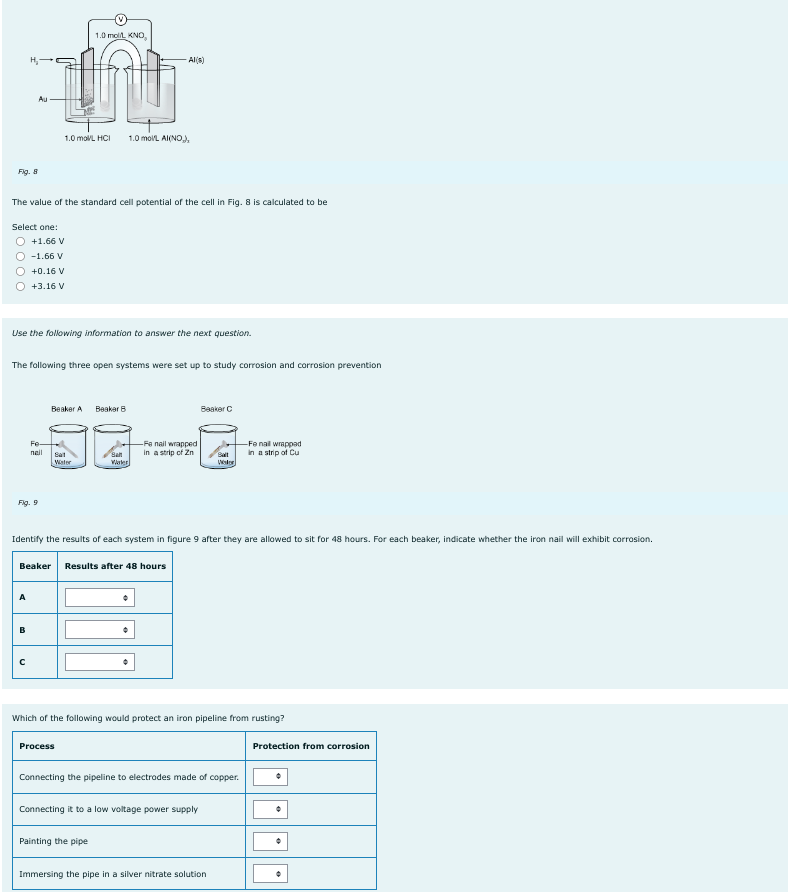
Transcribed Image Text:Fig. 8
Select one:
O +1.66 V
-1.66 V
+0.16 V
+3.16 V
Au
The value of the standard cell potential of the cell in Fig. 8 is calculated to be
Fig. 9
Use the following information to answer the next question.
The following three open systems were set up to study corrosion and corrosion prevention
A
Fe-
neil
B
1.0 mol/L HCI 1.0 mol/L AI(NO),
с
1.0 mol/L, KNO,
Beaker A
Sal
Water
Beaker Results after 48 hours
Process
Beaker B
-Al(s)
San
Water
Identify the results of each system in figure 9 after they are allowed to sit for 48 hours. For each beaker, indicate whether the iron nail will exhibit corrosion.
Painting the pipe
•
-Fe nail wrapped
in a strip of Zn
•
Beaker C
Which of the following would protect an iron pipeline from rusting?
Connecting it to a low voltage power supply
Balt
Wolor
Connecting the pipeline to electrodes made of copper.
-Fe naill wrapped
in a strip of Cu
Immersing the pipe in a silver nitrate solution
Protection from corrosion
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
