Figure 2 Two samples of Mg(s) of equal mass were placed in equal amounts of HCI(aq) contained in two separate reaction vessels. Particle representations of the mixing of Mg(s) and HCI(aq) in the two reaction vessels are shown in Figure 1 and Figure 2 above. Water molecules are not included in the particle representations. Which of the reactions will initially proceed faster, and why? The reaction in Figure 1, because the atoms of Mg are more concentrated than those in Figure 2 The reaction in Figure 1, because the Mg(s) in Figure 1 has a larger mass than the Mg(s) in Figure 2 The reaction in Figure 2, because more Mg atoms are exposed to HCI(aq) in Figure 2 than in Figure 1 The reaction in Figure 2, because the Mg(s) in Figure 2 has less surface area than the Mg(s) in Figure 1
Figure 2 Two samples of Mg(s) of equal mass were placed in equal amounts of HCI(aq) contained in two separate reaction vessels. Particle representations of the mixing of Mg(s) and HCI(aq) in the two reaction vessels are shown in Figure 1 and Figure 2 above. Water molecules are not included in the particle representations. Which of the reactions will initially proceed faster, and why? The reaction in Figure 1, because the atoms of Mg are more concentrated than those in Figure 2 The reaction in Figure 1, because the Mg(s) in Figure 1 has a larger mass than the Mg(s) in Figure 2 The reaction in Figure 2, because more Mg atoms are exposed to HCI(aq) in Figure 2 than in Figure 1 The reaction in Figure 2, because the Mg(s) in Figure 2 has less surface area than the Mg(s) in Figure 1
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter15: Equilibria Of Other Reaction Classes
Section: Chapter Questions
Problem 51E: Magnesium metal (a component of alloys used in aircraft and a reducing agent used in the production...
Related questions
Question
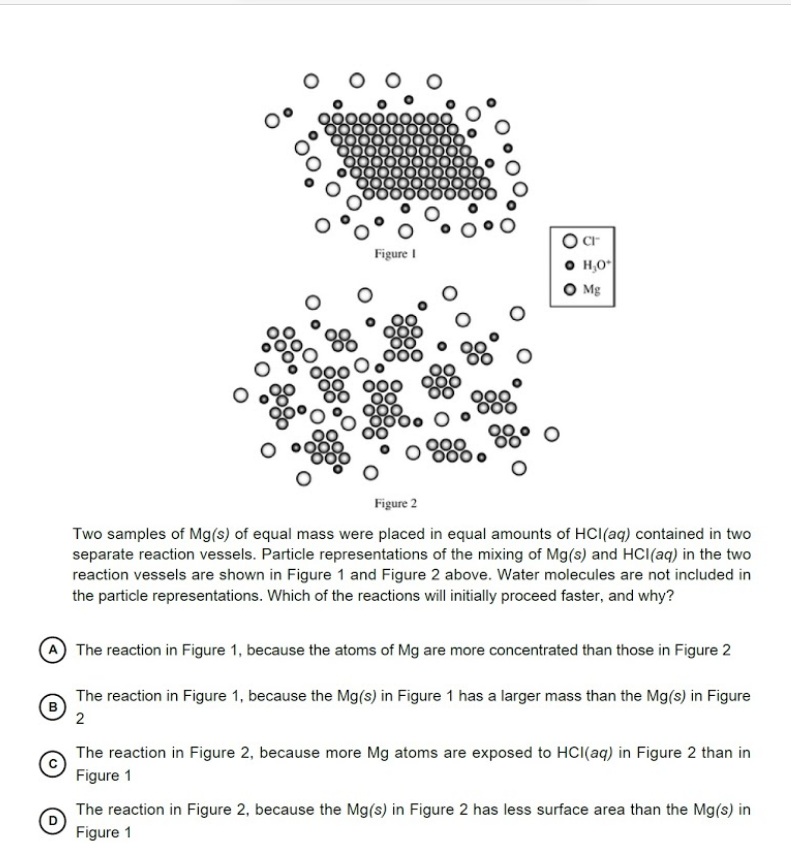
Transcribed Image Text:Figure I
H,O
O Mg
000
Figure 2
Two samples of Mg(s) of equal mass were placed in equal amounts of HCI(aq) contained in two
separate reaction vessels. Particle representations of the mixing of Mg(s) and HCI(aq) in the two
reaction vessels are shown in Figure 1 and Figure 2 above. Water molecules are not included in
the particle representations. Which of the reactions will initially proceed faster, and why?
(A The reaction in Figure 1, because the atoms of Mg are more concentrated than those in Figure 2
The reaction in Figure 1, because the Mg(s) in Figure 1 has a larger mass than the Mg(s) in Figure
2
The reaction in Figure 2, because more Mg atoms are exposed to HCI(aq) in Figure 2 than in
Figure 1
The reaction in Figure 2, because the Mg(s) in Figure 2 has less surface area than the Mg(s) in
Figure 1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning