Figure 3. Possible mechanism of bromination using Oxone as the oxidant Н. Br NaBr, Oxone но. ELOH, 0°C Figure 4. Reaction for this experiment: Bromination of vanillin with oxone as the oxidant to produce 5- bromovanillin. Useful Data: Molar masses (g/mol): Vanillin - 152.15 NaBr - 102.89 Oxone - 615.50 5-Bromovanillin - 231.04 Melting points: Vanillin - 81-83 °C 5-Bromovanillin - 164-166 °C %3. Experiment: First, set up an ice bath on a (UNheated) stir plate set on a ring stand. Next, weigh out approximately
Figure 3. Possible mechanism of bromination using Oxone as the oxidant Н. Br NaBr, Oxone но. ELOH, 0°C Figure 4. Reaction for this experiment: Bromination of vanillin with oxone as the oxidant to produce 5- bromovanillin. Useful Data: Molar masses (g/mol): Vanillin - 152.15 NaBr - 102.89 Oxone - 615.50 5-Bromovanillin - 231.04 Melting points: Vanillin - 81-83 °C 5-Bromovanillin - 164-166 °C %3. Experiment: First, set up an ice bath on a (UNheated) stir plate set on a ring stand. Next, weigh out approximately
Chapter1: Lewis Structures
Section: Chapter Questions
Problem 63EQ
Related questions
Question
Calculate the mass of sodium bromide you would want to measure assuming you actually measure out 0.50g of vanillin.
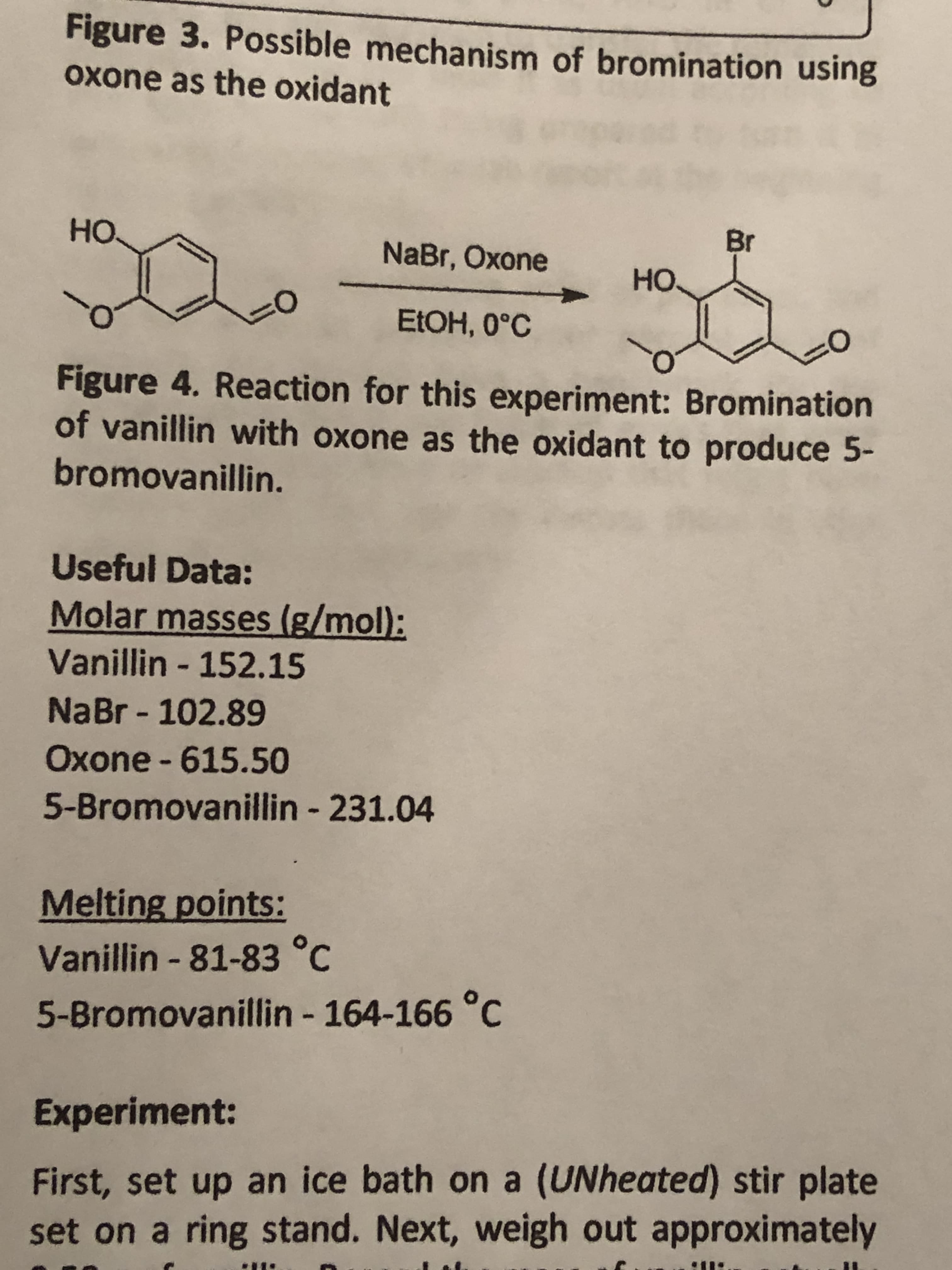
Transcribed Image Text:Figure 3. Possible mechanism of bromination using
Oxone as the oxidant
Н.
Br
NaBr, Oxone
но.
ELOH, 0°C
Figure 4. Reaction for this experiment: Bromination
of vanillin with oxone as the oxidant to produce 5-
bromovanillin.
Useful Data:
Molar masses (g/mol):
Vanillin - 152.15
NaBr - 102.89
Oxone - 615.50
5-Bromovanillin - 231.04
Melting points:
Vanillin - 81-83 °C
5-Bromovanillin - 164-166 °C
%3.
Experiment:
First, set up an ice bath on a (UNheated) stir plate
set on a ring stand. Next, weigh out approximately
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning



Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
