4) Benzene, CH, can be described as an aromatic substance and is a starting material for many organic processes as seen in the following. NO₂ benzene Compound A Compound 8 faming sulfuric acid SO,H Compound C a) Provide details of the bonding structure of benzene and sketch a diagram to show this. (3) b) For any one of these reactions above: i) State the reagents required and include the details of the formation of the reactive species required to start the reaction. (3) ii) Provide details of the reaction mechanism for this chemical process making clear any intermediates formed. (3)
4) Benzene, CH, can be described as an aromatic substance and is a starting material for many organic processes as seen in the following. NO₂ benzene Compound A Compound 8 faming sulfuric acid SO,H Compound C a) Provide details of the bonding structure of benzene and sketch a diagram to show this. (3) b) For any one of these reactions above: i) State the reagents required and include the details of the formation of the reactive species required to start the reaction. (3) ii) Provide details of the reaction mechanism for this chemical process making clear any intermediates formed. (3)
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.44P
Related questions
Question
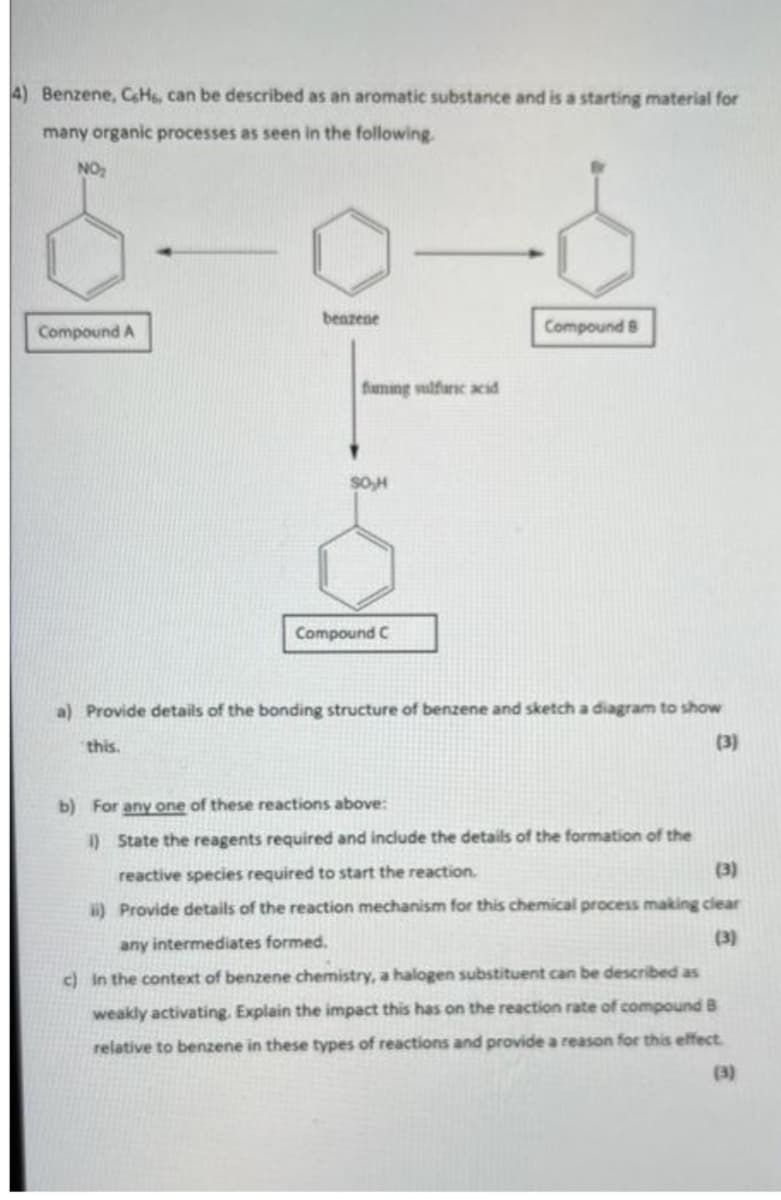
Transcribed Image Text:4) Benzene, CeH6, can be described as an aromatic substance and is a starting material for
many organic processes as seen in the following.
NO₂
benzene
Compound B
Compound A
Compound C
a) Provide details of the bonding structure of benzene and sketch a diagram to show
(3)
this.
b) For any one of these reactions above:
1) State the reagents required and include the details of the formation of the
(3)
reactive species required to start the reaction.
ii) Provide details of the reaction mechanism for this chemical process making clear
any intermediates formed.
(3)
c) in the context of benzene chemistry, a halogen substituent can be described as
weakly activating. Explain the impact this has on the reaction rate of compound B
relative to benzene in these types of reactions and provide a reason for this effect
(3)
faming sulfuric acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning
