Fill in the lable below with as appropriate to rank tne expected nydrophilicity eac illustrated molecules under the given conditions, with 1 being the most hydrophilic and 3 being the least hydrophilic. If any compound is expected to be hydrophobic under all conditions, write that in every box in that row. If any compound is expected to be hydrophilic under all conditions, write that in every box in that row. Lastly, justify your responses in every case with a brief and specific rationale. Molecule pH 2 pH 7 pH 13 H3C. CH3
Fill in the lable below with as appropriate to rank tne expected nydrophilicity eac illustrated molecules under the given conditions, with 1 being the most hydrophilic and 3 being the least hydrophilic. If any compound is expected to be hydrophobic under all conditions, write that in every box in that row. If any compound is expected to be hydrophilic under all conditions, write that in every box in that row. Lastly, justify your responses in every case with a brief and specific rationale. Molecule pH 2 pH 7 pH 13 H3C. CH3
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter6: Alkanes & Alkenes
Section: Chapter Questions
Problem 23CTQ
Related questions
Question
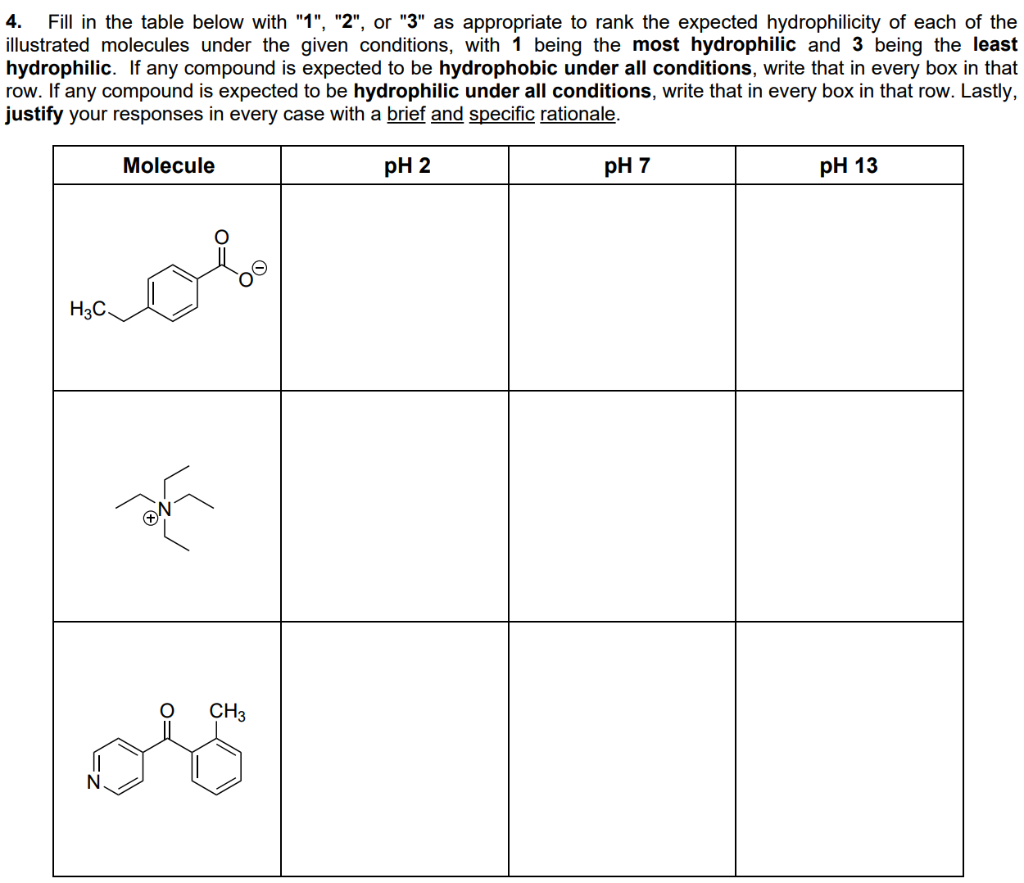
Transcribed Image Text:4. Fill in the table below with "1", "2", or "3" as appropriate to rank the expected hydrophilicity of each of the
illustrated molecules under the given conditions, with 1 being the most hydrophilic and 3 being the least
hydrophilic. If any compound is expected to be hydrophobic under all conditions, write that in every box in that
row. If any compound is expected to be hydrophilic under all conditions, write that in every box in that row. Lastly,
justify your responses in every case with a brief and specific rationale.
Molecule
pH 2
pH 7
pH 13
H3C.
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole