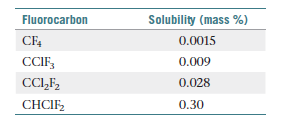
Fluorocarbons (compounds that contain both carbon
and fluorine) were, until recently, used as refrigerants.
The compounds listed in the following table are all gases
at 25 °C, and their solubilities in water at 25 °C and 1
atm fluorocarbon pressure are given as mass percentages.
(a) For each fluorocarbon, calculate the molality of a saturated
solution. (b) Which molecular property best predicts
the solubility of these gases in water: molar mass,
dipole moment, or ability to hydrogen-bond to water?
(c) Infants born with severe respiratory problems are sometimes
given liquid ventilation: They breathe a liquid that
can dissolve more oxygen than air can hold. One of these
liquids is a fluorinated compound, CF3(CF2)7Br. The solubility
of oxygen in this liquid is 66 mL O2 per 100 mL liquid.
In contrast, air is 21% oxygen by volume. Calculate the
moles of O2 present in an infant’s lungs (volume: 15 mL)
if the infant takes a full breath of air compared to taking
a full “breath” of a saturated solution of O2 in the fluorinated
liquid. Assume a pressure of 1 atm in the lungs.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 9 images






