Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 70QRT
Related questions
Question
Write the balanced equation for the following situation. List the reaction type.
Tell the amounts of every substance that remains in the container at the end of the reaction. Assume that all reactions go to completion. If only stoichiometry, tell how much of the excess reactant is used!!!!
Reaction Type:
a. Combination Reaction
b. Decomposition Reaction
c. Single Displacement / THIS IS ONE TYPE OF
d. Precipitation Reaction
e. Gaseous Reaction
f. Neutralization Reaction
g. Combustion Reaction
Follow the format used in the image.
5.92 g of sodium oxalate is reacted with 5.92 of calcium chloride
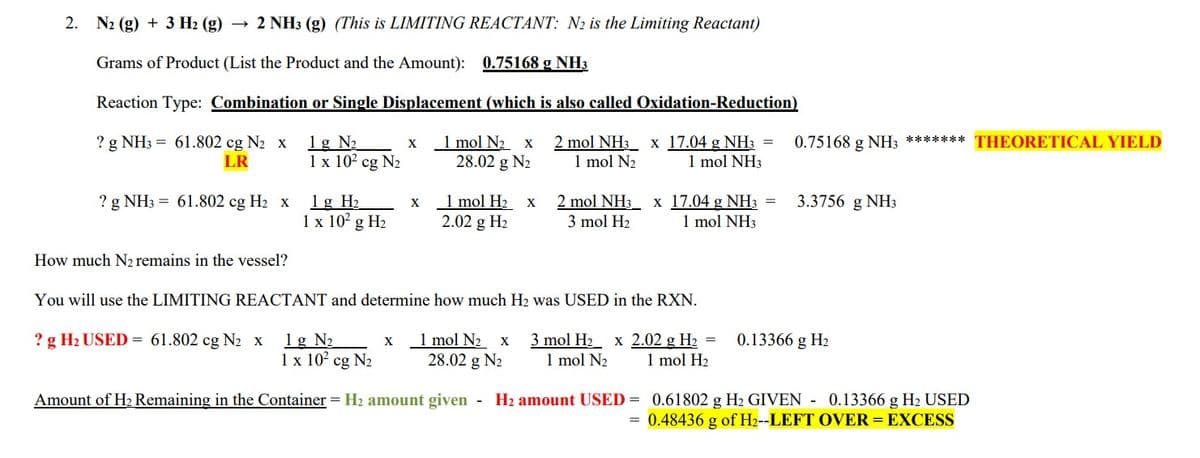
Transcribed Image Text:2.
N2 (g) + 3 H₂(g) → 2 NH3 (g) (This is LIMITING REACTANT: N₂ is the Limiting Reactant)
Grams of Product (List the Product and the Amount): 0.75168 g NH3
Reaction Type: Combination or Single Displacement (which is also called Oxidation-Reduction)
? g NH3 = 61.802 cg N₂ x 1g N₂
2 mol NH3 x 17.04 g NH3 =
1 mol N₂
1 mol NH3
LR
? g NH3 = 61.802 cg H₂ x
How much N₂ remains in the vessel?
1 x 10² cg N₂
1 g H₂
1 x 10² g H₂
X
X
1 mol N₂ x
28.02 g N₂
1 mol H₂
2.02 g H₂
x
2 mol NH3 x 17.04 g NH3 =
3 mol H₂
1 mol NH3
You will use the LIMITING REACTANT and determine how much H₂ was USED in the RXN.
=
0.75168 g NH3 *****
0.75168 g NH3
=
3.3756 g NH3
? g H₂ USED = 61.802 cg N₂ x 1g N₂
x 1 mol N₂ x 3 mol H₂ x 2.02 g H₂
1 x 10² cg N₂ 28.02 g N₂ 1 mol N₂ 1 mol H₂
Amount of H₂ Remaining in the Container = H₂ amount given - H₂ amount USED= 0.61802 g H₂ GIVEN - 0.13366 g H₂ USED
0.48436 g of H2--LEFT OVER = EXCESS
******* THEORETICAL YIELD
0.13366 g H₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning