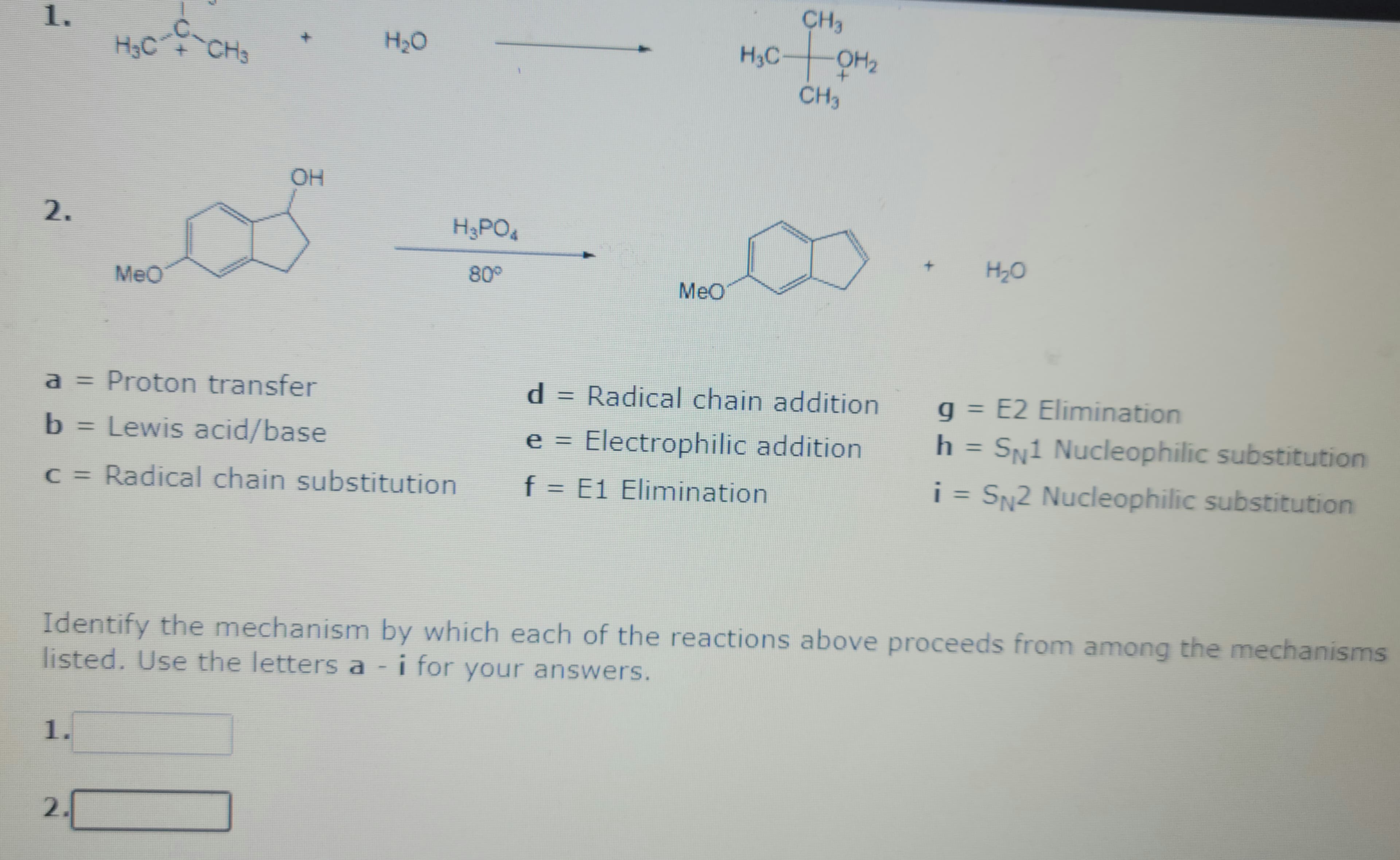
Q: 5.71 Identify the specific type of relationship between each of the following pairs of molecules (1.…
A: “Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…
Q: What is the major product for this reaction? (A) H₂ Pd (B) (C) ??? (D) What reagents would you use…
A:
Q: IDENTIFY THE OXIDATION NUMBER OF EACH ELEMENT IN THE GIVEN CHEMICAL FORMULA. -All answers to be…
A: The chemical formula of sodium chloride is NaCl
Q: b) Qax HO
A:
Q: 8. Complete the following reactions for the formation of acetals. Draw the structure of the product.…
A: 8. Acetal formation in presence of acid. 9. Hydrolysis of acetal in presence of H2O and acid
Q: What is the volume, in liters, of 4.00 moles of methane gas, CH4, at 14 °C and 1.20 atm?
A: We can solve this question using the Ideal Gas Equation : PV = nRT where , P = Pressure V = Volume n…
Q: Treatment of hydroxylamine (H2NOH) with an excess of Fe (III) results in the formation of N2O and…
A: Here we have to determine the molar concentration of NH2OH in the following given volumetric…
Q: Use the References to access important values if needed for this question. Determine the molecular…
A: Given, The molecular formula and empirical formula of the given structure is:
Q: 5. 6. 7. The next 3 questions refer to the diagram below. All cells are at standard state. Pb The…
A: #5: The standard reduction potential values of Pb and Zn are; Zn2+(aq) + 2e- →Zn(s) ; Eo = - 0.762 V…
Q: Identify reagents that can be used to complete the following transformation. OH O BH3-THF O 1)…
A:
Q: hich of the five different types of reactions is ur compound involved in? Include ormation about…
A:
Q: Which of the following compounds will display two triplets and a singlet in the ¹H NMR spectrum? □…
A:
Q: For the equilibrium system below, determine which direction the equilibrium will shift under each…
A:
Q: You were asked to estimate the pH of a 15mL of 0.09N of HCl when 16.9mL of 0.085N NaOH is added to a…
A:
Q: What descriptive term is applied to the type of diene represented by 2,4-hexadiene? O isolated diene…
A: Dienes contains two double bonds. There are following type of dienes - (1) Isolated Dienes - These…
Q: 1. What is the pH aft er adding 18 ml of 0.12N sodium hydroxide? 2.What is the pH at equivalence…
A: Solution: 4. Moles of NaOH used for 18 mL = molarity x volume/1000 = 0.12 x 18/1000 = 0.00216 moles…
Q: True or false: in a combustion reaction, ALL the products are compounds
A: A molecules is a group of two or more atoms that are held together by a chemical bond. While , a…
Q: Predict whether the following reaction shows an increase or a decrease in entropy B₂H6(g) 2 B(g) + 3…
A:
Q: Question 6 Calculate the percentage of MnO2 (MW 86.937 g/mol) in a mineral specimen if the 12…
A:
Q: According to the following reaction, how many moles of calcium chloride will be formed upon the…
A:
Q: esc 124 51 S63- Given the atomic symbol, identify the following: A: [Select] Z: [Select] # of…
A:
Q: At 25C, 0.00188 g of AgCl dissolves in one liter of water Write a balanced chemical equation What…
A:
Q: A 7.00 x 10-² M aqueous solution of aluminium chloride (AlCl3) is prepared for use in an experiment.…
A:
Q: Question 2: Some, but not all, compounds that feature a C—N bond are amines. (Think carefully about…
A: The given statement is true. i.e. All C---N bond are not amine.
Q: Calculate the standard Gibbs free energy (in kJ/mol) of the reaction below. (Use R = 8.314 J/mol-K;…
A:
Q: 2. At 2000.0°C, the Keq for the decomposition of carbon dioxide gas to carbon monoxide and oxygen…
A: Equilibrium constant is the ratio of concentration of products to concentration of reactants raised…
Q: t ALL types of intermolecular forces that exist among the following pure subs rubidium fluoride…
A:
Q: Check all of the compounds in the list below that are constitutional isomers. (Select all that…
A: Constitutional isomers are compounds having same molecular formula but different atom to atom…
Q: Reaction a: Br -NO₂ + (CH3)2N-H
A:
Q: A gas sample containing 12.0 g of He has a volume of 14.0 L. What will the volume be if 1.6 g of H2…
A:
Q: Calculate the reaction temperature of the electrochemical cell described below which measures a…
A: The given cell notation is: Cu(s) | Cu2+(0.81 M) || O2(g) (0.98 atm) | H+(0.047 M), H2O2(0.78 M) |…
Q: In a standard electrochemical cell, A4+ is reduced to solid A, while gaseous Z2 is oxidized to Z3-.…
A:
Q: Draw the HOMO of 1,3-butadiene,
A:
Q: Which of the following are examples of homogeneous mixtures? Choose all that ap Milk Mud Cooking oil…
A: To answer: Which of the following are examples of homogeneous mixtures? Choose all that apply Milk…
Q: Write the balanced neutralization reaction between Ca(OH), and HBr.
A:
Q: What is the molarity of a solution containing 2.75 moles of NaCl in 5.00 L of solution?
A: Definition of molarity- Molarity is the ratio of moles of solute to the volume of the solution.…
Q: Write the balanced equation for the following situation. In addition, list the reaction type. You…
A:
Q: What is the major organic product obtained from the following reaction? AICI, 'B) II C) III D) IV…
A: Given reaction is : What is the major organic product of the reaction ? Options are : Aromatic…
Q: What is the pH of a 2.72.7 M solution of HClO4?
A:
Q: Set up all the equations needed to use the systematic treatment of equilibria to solve for the pH of…
A: The systematic treatment of equilibrium requires a charge balance equation(CBE), mass balance…
Q: Calculate the standard Gibbs free energy (in kJ/mol) of the reaction below. (Use R = 8.314 J/mol-K;…
A:
Q: A solution of sodium thiosulfate was standardized by dissolving 0.3815 g KIO3 (214.00 g/mol) in…
A:
Q: A chemist prepares a solution of sodium hyposulfate (Na₂S₂O3) by measuring out 200. μmol of sodium…
A: A homogeneous mixture of two or more substances is known as a solution. The species which present in…
Q: Which of the following are examples of impure substances? Choose all that apply. D Heterogeneous…
A: Substances can be classified as pure and impure substances. There are given different substances,…
Q: compound Reagents HX a. b. HBr. H₂O₂, hv c. H₂O, H₂SO4 d. X₂ e. H₂. Pd X₂, H₂O 9 Oso, then NaHSO, f.…
A: substitution reaction is chemical reactions in which an atom , or group in a molecule is replaced…
Q: Zn²+ (aq) + Al(s) → Zn(s) + Al³+ (aq) 2+ 3Zn** (aq) + Al(s) Zn(s) + 2 A1³+ (aq) Zn(s) + 3A1³+ (aq)…
A: Redox reaction are those in which both oxidation and reduction reaction occurs simultaneously.
Q: chlorine to produce nitrogen and hydrogen chloride gas. How many litres of Gaseous ammonia reacts…
A:
Q: The following are unbalanced equations. They show the kinds of reactants and products involved but…
A: The correct reaction type of the above reaction is given below
Q: present and mechanism of carbonic anhydrase II. Briefly explain each step
A:
Q: Treatment of hydroxylamine (H₂NOH) with an excess of Fe (III) results in the formation of N₂O and an…
A: Given, 2H2NOH +4 Fe3+→N2O(g) +4Fe2++14H++H2O (i) Volume of K2Cr2O7=12.57 mL Molarity of…
Step by step
Solved in 2 steps with 2 images

- I have this task in organic chemistry (book: Brown's introduction to organic chemisty, global edition). Task 10:42. In (a) I have to tell what the funcion of K2CO3 is in step 1. Is it that CO32- take the hydrogen atom in 1-napthol? Will it then be a SN2 mechanism? In (b) I have to name the amine used in step 2 to form Propanolol. But I can't really find out how to come up with an amine that will make that reaction. Here are two pictures of the task:Orgonic Chemistry II: The answer is writtten as followed. But I Need Explanation. My question is that: Can I siwtch reagent 2 to reagent 1? for example reagent 1 is Cl2,Fecl3, can I changed it to reagent 2??? Why and why not???Compound X is insoluble in cold KMnO4, Br2 in CCl4, and conc. H2SO4. Compound X is most likely: a. an alkane b. none of these c. an alkene d. an alcohol e. an alkyl halide Indicate which of the ff. statements regarding nucleophilicity is incorrect. F- is more nucleophilic, hence, more reactive towards methyl iodide than Cl-. Second row elements are more nucleophilic than first row elements of comparable basicity. The rate of SN2 reaction may be markedly affected by the nucleophilicity of the attacking atom. Nucleophilicity is the affinity of a nucleophile to an electrophilic carbon Which of the following alkynes can be deprotonated by NaNH2 in liquid NH3? 3-Methylhex-2-yne Pent-2-yne 3-Methylbutyne none of these Hex-3-yne
- Given this organic synthesis, is there any limiting and excess reagents? also describe the procedure is run, how is the reaction monitored?Is the order fo addition important? N-ETHYLALLENIMINE[Aziridine, 1-ethyl-2-methylene-] Submitted by Albert T. Bottini and Robert E. Olsen1.Checked by Thomas H. Lowry and E. J. Corey. 1. ProcedureCaution! This preparation should be carried out in a good hood to avoid exposure to ammonia. The operator should wear rubber gloves and protective goggles because 2-haloallylamines and ethylenimines can cause severe skin and eye irritation. A 2-l. three-necked flask is fitted with a sealed mechanical stirrer, a gas-inlet tube, and a dry ice condenser protected from the air by a soda-lime drying tube (Note 1). The system is flushed thoroughly with dry ammonia, and 32.8 g. (0.84 mole) of sodium amide (Note 2) is added to the flask. The system is again flushed with ammonia, the condenser is provided with dry ice covered by acetone, and 1.2 l. of liquid…Describe every aspect of the procedure clearly and explicitly. Include temperatures, appearance of the reaction (include pictures!). How is the reaction monitored? Is the order of addition important? 4,4'-DIBROMOBIPHENYL [Biphenyl, 4,4'-dibromo-] Submitted by Robert E. Buckles and Norris G. Wheeler1. Checked by R. S. Schreiber, Wm. Bradley Reid, Jr., and Robert W. Jackson. 1. Procedure In a 15-cm. evaporating dish is placed 15.4 g. (0.10 mole) of finely powdered biphenyl (Note 1). The dish is set on a porcelain rack in a 30-cm. desiccator with a 10-cm. evaporating dish under the rack containing 39 g. (12 ml., 0.24 mole) of bromine. The desiccator is closed, but a very small opening is provided for the escape of hydrogen bromide (Note 2). The biphenyl is left in contact with the bromine vapor for 8 hours (or overnight). The orange solid is then removed from the desiccator and allowed to stand in the air under a hood for at least 4 hours (Note 3). At this point, the product weighs…Organic Chemistry 1. Can you please explain all three answer choices ? Why is it ! and III, and not II ? Thank you
- Solution:- 3. Determine the amount, in of a (2.20 M s olution of dichloromethane needed to completely react with 15.72g cyclohexene to give 1,2-dibromocuclohexanw. Assume 12% excess is needed in order to react completely. a . How much 1,2 -dibromocyclohexane would theoretically be produced ? c. How many ML of the 2.20 M Br2 solution are required?Tunicates are marine animals that are called "sea squirts" because when they are taken out of water, they tend to contract and expel seawater. Lepadiformine is a cytotoxic agent (toxic to cells) isolated from a marine tunicate. During a recent synthesis of lepadiformine, the investigators observed the formation of an interesting by-product (3) while treating diol 1 with a reagent similar in function to PBr3 (J. Org. Chem. 2012, 77, 3390–3400):In depth explanation of each, please.

