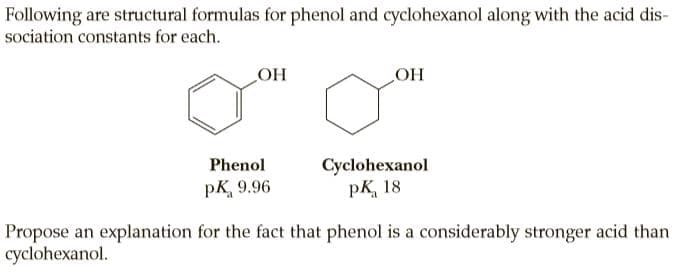
Following are structural formulas for phenol and cyclohexanol along with the acid dis- sociation constants for each. OH НО Cyclohexanol pK, 18 Phenol pK, 9.96 Propose an explanation for the fact that phenol is a considerably stronger acid than cyclohexanol.
Q: 4. a) Place the following compounds in order of increasing acidity of their most acidic proton. b)…
A: Solution- The acid is differentiate with their acidity. The most acidic proton is more acidic.
Q: The compound obtained by treating propan-1-ol with PCC followed by excess of ethanol in presence of…
A:
Q: The structure of an ester could either be A or B: CH3 O O CH3 T || -C-OCH3 CH3-C CH3-C-0-C-CH3 CH3…
A: An instrumentational method that is used to determine the structure of a compound by analyzing its…
Q: Arrange these compounds in order of increasing acidity: 2,4-dichlorophenol, phenol, cyclohexanol.
A: 2,4-dichlorophenol Phenol Cyclohexanol
Q: Show how you would synthesize each compound from benzene, toluene, or phenol using the following…
A: Answer of the question given below,
Q: Propose a mechanism for the steam hydrolysis of pulegone to the compounds shown.
A: Hydrolysis – Addition of water in a given molecule is called hydrolysis Hydrolysis is simply the…
Q: resonance and inductive effects if present.
A: Both phenol and benzene are nucleophilic in nature due to different reasons. Both, inductive and…
Q: a) Para, ortho and meta nitrophenol are constitutional isomers of one another that all vary in the…
A: Nitro phenols are the derivative of benzene having formula OHC6H5-X(NO2)x. There are 3 types of…
Q: Arrange the following compounds in decreasing order of their acid strength 2, 4, 6-trinitrophenol 3…
A: Alcohols can be defined as the organic compounds that contain hydroxyl group is attached to carbon…
Q: Rank the following compounds in order of increasing λmax:
A: Wavelength and conjugation are directly related to each other. Conjugation means the presence of a…
Q: 3. p-Nitrobenzyl alcohol is more acidic than benzyl alcohol but p- methoxybenzyl alcohol is less…
A: Acid: It is the ability of a compound to donate protons to other species. The structure of…
Q: Consider the following reaction to form compound C. OH H3C cat. Ph H OCN В Ph A a) Indicate the most…
A: More electrophilic centre will react with cat. Cyanide, Because CN- is a nucleophile.
Q: What generalization trends may be extracted from the following series in terms of solubility with…
A: Acid base reaction is faster than other reaction so Base will react first with an acid. Now in set…
Q: B) Rank the following compounds toward increasing acidity, , why? 2) 1,3-benzoqounoin…
A: The acidic order depends on two factor 1) how fast the given compound release H+ ions 2) the…
Q: Although benzene itself absorbs at 128 ppm in its 13C NMR spectrum, the carbons of substituted…
A: Given: Although benzene itself absorbs at 128 ppm in its 13C NMR spectrum, the carbons of…
Q: From cyclohexane and toluene as a carbon base, synthesize : 3-(3-cyclohexenyl)benzoic acid
A:
Q: 1. Which compound has the lowest boiling point? A. 2,6-Dibromophenol B. Hexan-2-one C. Octan-1-ol…
A: Boiling point of an organic compound is the temperature at which an attractive force between the…
Q: Treatment of alcohol A (molecular formula C5H12O) with CrO3, H2SO4, and H2O affords B with molecular…
A:
Q: A. Reaction with Nitrogen Compounds; Write whether the following aldehydes and ketones are positive…
A: A. The aldehydes and ketones considered are not positive species. But have polarized carbonyl bonds…
Q: (a) How will you convert the following :(i) Propanone to Propan-2-ol (ii) Ethanal to…
A: Oxidation It is the process of gaining of oxygen Different oxidizing agents are; alkaline KMnO4, Br2…
Q: Which among the following reagent in the choices can be utilized to differentiate propionaldehyde…
A:
Q: Starting exactly with any acid chloride with exactly with 5 carbon atoms, and using appropriate…
A: A question based on introduction to organic chemistry that is to be accomplished.
Q: When phenylbenzoate is heated with aqueous acid solution, the products formed are: A. 2 moles of…
A:
Q: Write the formula of reagents used in the following reactions:(i) Bromination of phenol to…
A: A substance added in a chemical reaction in order to see if a chemical reaction occurs is called a…
Q: Friedel- Crafts Acylation correlate the boiling points of toluene and 1-(p-tolyl) ethanone with…
A: Friedel- Crafts Acylation: Electrophilic aromatic substitution reactions occur between the…
Q: In acid-base equilibria, the presence of an electron withdrawing substituent on the R- group of the…
A: The nature of alcohols (R-OH) is acidic. After the removal of proton (deprotonation), alcohol forms…
Q: Draw a structural formula for the product formed by treating butanal with each reagent. (a)…
A: Since the question you have posted contains multiple subparts, we can solve only first three…
Q: The Claisen condensation converts two molecules of an ester into a B-keto ester. The reaction starts…
A:
Q: Carbon NMR Spectrum A has peaks at & 71, 40, 19, and 12. Spectrum B has peaks at 8 72, 38, 31, 29,…
A: The solution is as follows:
Q: Explain the following behaviours :(i) Alcohols are more soluble in water than the hydrocarbons of…
A: (i) Alcohols are soluble in water as they form hydrogen bonds with water molecules because of…
Q: Show how to convert cyclopentanone to these compounds. In addition to cyclopentanone, use any other…
A: Reduction reactions the gaining of an electron, a gain of hydrogen, loss of oxygen. Cyclopentanone…
Q: Using ChemDraw Predict 1H NMR spectra of acetophenone and the protonated acetophenone
A: Using chem draw we easily predict 1H NMR and 13C NMR. This helps us to cross-check obtained spectra.
Q: Aldehydes and ketones could be distinguished using Tollens test. What structural and/or molecular…
A: Acidity is defined as the ease with which a molecule can donate protons. if it can donate PROTONS…
Q: The 1H NMR spectrum of N,N-dimethylformamide shows three singlets at 2.9, 3.0, and 8.0 ppm. Explain…
A: To explain this, 1) Draw the resonance structure of N,N-dimethylformamide. 2) Check whether rotation…
Q: Explain WHY/HOW all please - Propanol CANNOT protonate ethylamine - 3-methylheptan-3-ol CANNOT be…
A: Since you have asked a question with multiple subparts, we will answer only first three subparts for…
Q: Write reactions of 3-methylpentanol with the following reagents: a. Na b. H2SO4 conc. t = 0°C c.…
A: Structure of 3-methylpentanol is:
Q: Suppose you have a mixture of these three compounds. Devise a chemical procedure based on their…
A: The aromatic nitro compounds are depicted by Ar-NO2 and the nitro substituent deactivates the ring…
Q: Provide a stepwise synthesis for benzyl alcohol to sodium benzoate with reagents KMnO4 and NaOH
A:
Q: In the following pairs of compounds, which is the most acidic? Benzoic acid and 4-nitrobenzoic acid…
A:
Q: Identify the most and the least basic compound in each of the following sets. Leave the remaining…
A: The basicity of the compound depends on several factors available. For example the electronegative…
Q: Please indicate which is the most acidic compound in each of the following pairs: thank you…
A: Most acidic compound in each of the following pairs =? 1) p-Nitrophenol and m-nitrophenol 2)…
Q: match compound e based on its reaction to the various reagents? ethanol acetic acid benzaldehyde…
A: Tollen's test is used for the detection of aldehyde in the presence of ketones. Aldehydes give…
Q: What peaks are expected in the IR spectrum and NMR (C-NMR and P-NMR) spectrum of triphenyl triethyl…
A: Here we consider the decoupled NMR , where peaks do not further splitted .
Q: Arrange the following compounds in the increasing order of their acid strength: p-cresol,…
A: The following compounds in the increasing order of their acid strength have to be given. p-cresol,…
Q: Explain the solubility behavior of each compound in water with relevance to their structure. a.…
A:
Q: What is the intermédiate state of an acid-base catalysis during conversion of DHAP to GAP called,…
A:
Q: Melting Point Range Identity of Aldehyde Identity of Ketone 179-181 C Cinnamaldehyde Cyclohexanone…
A: In Aldol reaction enolate of an aldehyde or ketone reacts with another aldehyde or ketone to form…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- Arrange the following compounds in the increasing order of their acid strength: p-cresol, p-nitrophenol, phenolExplain the following observations :(i) The boiling point of ethanol is higher than that of methoxymethane.(ii) Phenol is more acidic than ethanol.(iii) o- and p-nitrophenols are more acidic than phenol.Phenols are less acidic than carboxylic acids, with values of pKa around 10. Phenols aredeprotonated by (and therefore soluble in) solutions of sodium hydroxide but not by solutions of sodium bicarbonate. Explain how you would use extractions to isolate the three purecompounds from a mixture of p-cresol (p-methylphenol), cyclohexanone, and benzoic acid.
- Explain the following behaviours :(i) Alcohols are more soluble in water than the hydrocarbons of comparable molecular masses.(ii) Ortho-nitrophenol is more acidic than ortho-methoxyphenol.The following three derivatives of succinimide are anticonvulsants that have found use in the treatment of epilepsy, particularly petit mal seizures. Q. Of these three anticonvulsants, one is considerably more acidic than the other two. Which is the most acidic compound? Estimate its pKa and account for its acidity. How does its acidity compare with that of phenol? with that of acetic acid?Provide a stepwise synthesis for benzyl alcohol to benzoic acid (including arrow-pushing mechanisms) Steps: benzyl alcohol - sodium benzoate - benzoic acid Kindly show all the arrow push
- Rank the compunds in the order of increasing acidity.a. Phenol, p-methylphenol, p-(trifluoromethyl)phenolb. Benzyl alcohol, phenol, p-hydroxybenzoic acid Illustrate and explain your answerMost alkyl bromide are water-insoluble liquids. But when the following alkyl bromide Awas first isolated, its high melting point of 203oC (solid at room temperature) and its water solubility led its discoverers to comment that it behaves more like a salt (hint: like NaCl). Explain the salt-like behavior of compound A in under thirty words.p-Nitrobenzyl alcohol is more acidic than benzyl alcohol but p-Methoxybenzyl alcohol is less acidic. Illustrate your explanation.
- 1. Arrange the following compounds in order of increasing acidity and explain the reason for the arrangement: Ethanoic acid, Butanoic Acid, Benzoic Acid, Salicyclic acid. 2. Arrange the test samples in order of increasing reactivity with sodium bicarbonate? Explain briefly. -Ethanoic acid, Butanoic acid, Benzoic acidExplain the solubility behavior of each compound in water with relevance to their structure. a. Acyl compounds in water-acetic acid (clear colorless solution)-benzoic acid (turbid, cloudy solution)-sodium benzoate (clear colorless solution)(a) Give chemical tests to distinguish between :(i) Propanol and propanone (ii) Benzaldehyde and acetophenone(b) Arrange the following compounds in an increasing order of their property as indicated :(i) Acetaldehyde, Acetone, Methyl tert-butyl ketone (reactivity towards HCN)(ii) Benzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)(iii) CH3CH2CH (Br) COOH, CH3CH (Br) CH2COOH, (CH3)2CHCOOH (acid strength)

