In acid-base equilibria, the presence of an electron withdrawing substituent on the R- group of the alcohols increase the acidity of the alcohol by stabilizing the ROH group by decreasing the stability of the RO- group by increasing the stability of the RO- group decreasing the stability of the ROH group
In acid-base equilibria, the presence of an electron withdrawing substituent on the R- group of the alcohols increase the acidity of the alcohol by stabilizing the ROH group by decreasing the stability of the RO- group by increasing the stability of the RO- group decreasing the stability of the ROH group
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter10: Alcohols
Section: Chapter Questions
Problem 10.49P
Related questions
Question
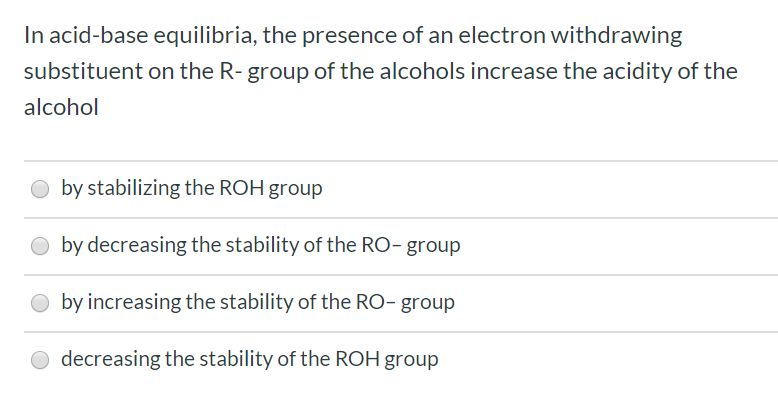
Transcribed Image Text:In acid-base equilibria, the presence of an electron withdrawing
substituent on the R- group of the alcohols increase the acidity of the
alcohol
by stabilizing the ROH group
by decreasing the stability of the RO- group
by increasing the stability of the RO- group
decreasing the stability of the ROH group
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
