The analgesic meperidine (Demerol) was developed in the search for analgesics with- out the addictive effects of morphine. As shown in these structural formulas, it repre- sents a simplification of morphine's structure. H.C. H,C. CH3 (redraw) НО OH Morphine Meperidine Meperidine is prepared by treating phenylacetonitrile with one mole of bis(N-2- chloroethyl)methylamine (a nitrogen mustard) in the presence of two moles of sodium hydride to give (A). Refluxing (A) with concentrated sodium hydroxide followed by neutralization of the reaction mixture with dilute HCl gives (B). Treating (B) with ethanol in the presence of one equivalent of HCl gives meperidine as its hydrochloride salt. N-CH, 1. NaOH C13H16N2 2 NaH 2. HCI (A) Phenylacetonitrile ELOH, HCI C13H1,NO, C5H2NO, · HCI (В) Meperidine hydrochloride
The analgesic meperidine (Demerol) was developed in the search for analgesics with- out the addictive effects of morphine. As shown in these structural formulas, it repre- sents a simplification of morphine's structure. H.C. H,C. CH3 (redraw) НО OH Morphine Meperidine Meperidine is prepared by treating phenylacetonitrile with one mole of bis(N-2- chloroethyl)methylamine (a nitrogen mustard) in the presence of two moles of sodium hydride to give (A). Refluxing (A) with concentrated sodium hydroxide followed by neutralization of the reaction mixture with dilute HCl gives (B). Treating (B) with ethanol in the presence of one equivalent of HCl gives meperidine as its hydrochloride salt. N-CH, 1. NaOH C13H16N2 2 NaH 2. HCI (A) Phenylacetonitrile ELOH, HCI C13H1,NO, C5H2NO, · HCI (В) Meperidine hydrochloride
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.63P
Related questions
Question
Propose a mechanism for the formation of (A)
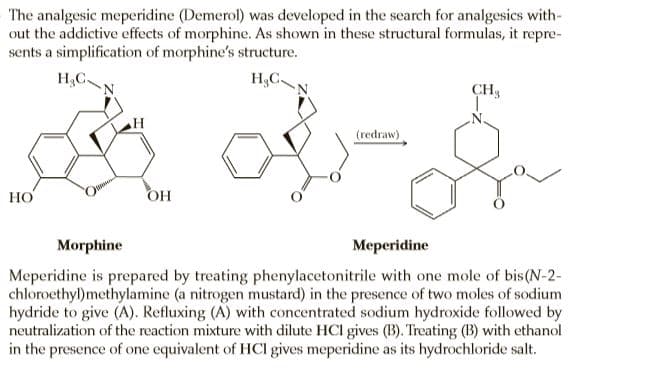
Transcribed Image Text:The analgesic meperidine (Demerol) was developed in the search for analgesics with-
out the addictive effects of morphine. As shown in these structural formulas, it repre-
sents a simplification of morphine's structure.
H.C.
H,C.
CH3
(redraw)
НО
OH
Morphine
Meperidine
Meperidine is prepared by treating phenylacetonitrile with one mole of bis(N-2-
chloroethyl)methylamine (a nitrogen mustard) in the presence of two moles of sodium
hydride to give (A). Refluxing (A) with concentrated sodium hydroxide followed by
neutralization of the reaction mixture with dilute HCl gives (B). Treating (B) with ethanol
in the presence of one equivalent of HCl gives meperidine as its hydrochloride salt.
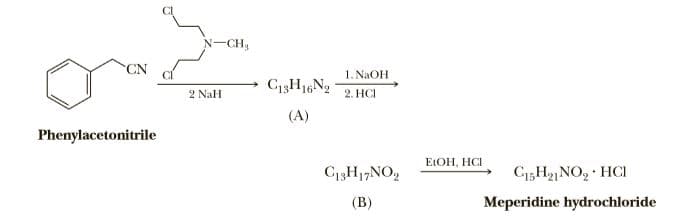
Transcribed Image Text:N-CH,
1. NaOH
C13H16N2
2 NaH
2. HCI
(A)
Phenylacetonitrile
ELOH, HCI
C13H1,NO,
C5H2NO, · HCI
(В)
Meperidine hydrochloride
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
