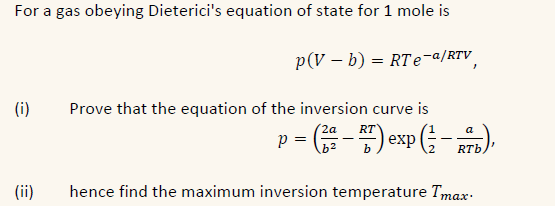
For a gas obeying Dieterici's equation of state for 1 mole is p(V - b) = RTe-a/RTV (i) (ii) Prove that the equation of the inversion curve is 2a RT a p = (22-27) exp (b), hence find the maximum inversion temperature Imax.
Q: Write the common (not systematic) name of each organic molecule. Hint: your answer should have more…
A: Writing the common name of the given compound given as below.
Q: Liquid hexane (CH₂(CH₂) CH₂) v will react with gaseous oxygen (0₂) to produce gaseous carbon dioxide…
A:
Q: Mixture of liquid toluene (T) and benzene (B) form ideal solutions at 60˚C. The vapour pressures of…
A: To determine the mole fraction of B in a liquid mixture which has a vapor pressure of 0.400 bar at…
Q: Which of the following DOES NOT describe chirality? mirror images are superimposable should have…
A: The statement which does not describe Chirality given below.
Q: CH₂CH3 H3C-C-CH3 :Br: NA H H3C For the reaction above, draw curved arrows to show the movement of…
A: Draw the curved arrows that depict the mechanisms by which the reactions occur is one of the most…
Q: Design a reasonable synthesis for the transformation shown Br
A: Three and four-membered cyclic rings usually undergo ring expansion through bond migration due to…
Q: Calculate the pH in the titration of 100.0 mL, of 0.20 M HCIO (K = 4.0 x 10^-8) with 0.20 M KOH…
A: Given that, Volume of HClO=100.0mL Concentration of HClO=0.20M Ka=4.0*10-8 Concentration of…
Q: Assign oxidation states to all elements in the reaction below and identify which species below. 2 As…
A:
Q: 1 7. Routine analysis of a water sample provides the following concentrations (in M). a) What is the…
A: Ionic strength is a measure of the concentration of ions in a solution and their ability to conduct…
Q: 1 Consider a 1.0 M HNO2 solution. Part A: What is the pH of this solution? Hint: The K₂ of HNO2 is…
A: The question is based on the concept of chemical equilibrium. we need to calculate percentage degree…
Q: How many O₂ molecules are needed to make 6 H₂O molecules? Explain how you know or show how you…
A: A question based on stoichiometry. An unbalanced equation is prescribed for which the appropriate…
Q: A solution is prepared by mixing 455 mL of 0.400 M NH 3 and 280 mL of 0.400 M HCl. Assuming that the…
A:
Q: Nickel(II) sulfate, used for nickel plating, is prepared by treatment of nickel(II) carbonate with…
A: Molar mass of NiCO3=118.702g/mol Molar mass of H2SO4=98.079g/mol Molar mass of NiSO4=154.75g/mol…
Q: Answer the questions in the table below about the shape of the sulfur pentafluoryl (SF) cation. What…
A: We have to tell the shape of SF5+ and smallest bond angle
Q: A mixture of gases has 0.3000 mol of CO2, 0.3190 mol of SO2 and 0.3500 mol of water vapor. The total…
A: Given that, a gas mixture having 0.3000 mol of CO2, 0.3190 mol of SO2, 0.3500 mol of water vapor.…
Q: When the concentration of H 3 O + in an aqueous solution is 5.6 x 10^-10 M, then the concentration…
A:
Q: 7. Being bored during the endless Wisconsin winter, you start digging in your back yard and discover…
A: 7. Given,Initial mass of the material = 400 gAfter 8 days mass of the material left = 250…
Q: Complete the table below by writing the symbols for the cation and anion that make up each ionic…
A: Here, we have to complete the given table by writing the symbols for each cation and anion that make…
Q: One day in lab, while taking apart a complicated distillation apparatus, your friend Reuben (an…
A: Balanced chemical equation: Balanced chemical equation can be divided as the reaction in which…
Q: reaction container I S 9. [McQuarrie 19-56] Consider the reaction: 2 ICI(g) 12(g) + Cl₂(g) a. The…
A: The question is based on the concepts of chemical equilibrium. we need to calculate equilibrium…
Q: Jon walked 89 feet in 11.5 seconds. What is his speed in miles per hour? (1mi)=(5280 ft)
A: Given , Jon walked = 89 feet Time = 11.5 seconds
Q: Draw the product of this series of reactions. 1. 1 equivalent of NaNH₂ 2. CH₂CH₂Br 3. H₂/ Lindlar's…
A: Organic reactions are those in which organic reactant react to form organic products. In the given…
Q: Macmillan L Cl name: Incorrect MAR OH CI CI
A: The question is based on the concept of IUPAC naming. we need to identify the IUPAC name of the…
Q: Calculate the formula mass of mg(clo3)2
A: Formula mass of a substance can be calculated by combining the average atomic masses of each atom…
Q: Consider the malate dehydrogenase reaction from the citric acid cycle. Given the listed…
A:
Q: Rainwater is slightly acidic due to dissolved CO₂. Use the following data to calculate the pH of…
A: l
Q: 2. Briefly explain why it is essential that none of the yeast comes into contact with the H₂O2…
A: H2O2 should come into contact with yeast before the stoppers are firmly placed as the oxygen…
Q: 3. Fill out the missing boxes in the table below, after referring to the object in Photo X (press on…
A: The question is based on the concept of mathematical calculations. we need to calculate volume and…
Q: Consider the following acid-base equilibrium. HC6H5O+ C4H7O2 C6H50 + HC4H7O2 The position of the…
A:
Q: For each row in the table below, decide whether the pair of elements will form a molecular compound…
A: We have to determine if the given elements will form a molecular compound and fill the given table
Q: nterpret the CNMR and HNMR
A: 1H-NMR spectroscopy is mainly used for the identification of structure of unknown compound. From the…
Q: The thermal decomposition of nitryl chloride is proposed to occur by the following mechanism: step 1…
A: Slow step is the rate determining step
Q: Calculate the pH in the titration of 100.0 mL, of 0.20 M HCIO (Ka = 4.0 x 10^-8with 0.20 M KOH after…
A: In acid base reactions the products formed are salt and water, hence these reactions are also…
Q: Is it 10 or 3.55
A: 4. Recall the given reaction, CaMgCO32 ↔ Ca2+ + Mg2+ + 2 CO32-at 25 °C…
Q: Please help what are special rules or laws to predict predominant products for alcohols or et
A: Here's a chart summarizing some special rules or laws to predict predominant products for alcohols…
Q: hello i need help with this question Assuming standard conditions, answer the following questions.…
A: “Since you have posted multiple subparts questions, we will provide the solution only to the first…
Q: Draw the structure of the compound that is consistent with the ¹H NMR. (Assume that long-range…
A: Question is Based on the concept of organic spectroscopy. We need to analyse the spectre and…
Q: Find the Kp for the reaction below given that the Kc is 10.5 at 220 °C. CO (g) + 2H2 (g) = CH3OH(g)
A:
Q: nd, er Di 'H-NMR 13 C-NMR Aldehyde 10 No neighbors -singlet, IH 2 neighbor triplet, IH Imeighbor…
A: The question is based on organic spectroscopy. we need to analyse the spectra and identify the…
Q: A chemist adds 430.0 mL of a 2.89 mol/L calcium bromide (CaBr₂) solution to a reaction flask.…
A: To calculate the mass of calcium bromide added to the reaction flask, we need to use the following…
Q: 5. Provide a step-by-step reaction mechanism for the following transformation. You must show all…
A: Ketals are prepared by reacting a ketone with alcohol in the presence of an acid catalyst. In this…
Q: 5. 6. 7. Write the Lewis Structure for SC1₂ (S is the center atom) Write the Lewis Structure for…
A:
Q: What is the coefficient for O2 when it is balanced? C6H5CH3(I) + O2(g) -> CO2(g) + H2O(g)
A:
Q: Macmillan Learning Use the van der Waals equation of state to calculate the pressure of 2.80 mol of…
A: The van der Waals equation of state for real gases is given by, P+an2V2V-nb=nRTwhere,P=Pressure…
Q: Which salts will be more soluble in an acidic solution than in pure water? ✓Zn(OH)₂ Agl CaCO3 BaSO4…
A: Answer: The salt whose anion reacts with H+ ion to form a new compound then that salt will be more…
Q: Given chemicals Hydrochloric acid, 2.0 M HCl Sodium hydroxide, 2.0 M NaOH ● Ammonium chloride, 2.0 M…
A: Answer: Moles are an extensive property therefore chemical equations can be added or subtracted…
Q: Starting with benzene and using any other necessary reagents of your choice, design a synthesis the…
A: Given that, a transformation is shown below We have to carry out the above transformation.…
Q: At 400 K oxalic acid decomposes according to the reaction: H_C,O4(g)—CO,(g)+HCOOH(g) In three…
A: The given chemical reaction is H2C2O4(g) →CO2(g) + HCOOH(g)
Q: Give the IUPAC name for the following compound. Be sure to answer all parts. (select) (select)…
A: Rules for IUPAC naming always consider the longest alkane carbon as the parent name for naming a…
Q: Fats have a high amount of stored energy because once ingested, they quickly and easily dissolve in…
A: Fats, also known as lipids, are a type of macronutrient that play a variety of important roles in…
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

- 9. Calculate the Go in kJ/mol. 3Fe2+ (s) + 2Cr(aq) ⟶2Cr3+(aq) + 3Fe (s); Eocell=0.30 V Group of answer choices -170 +170 +87 +58 -195 10. Calculate Ecell in volts. Zn(s)|Zn2+(2.50x10-4M)||Sn2+(1.50M)|Sn(s) Eocell=+0.624 V Group of answer choices 0.736 0.635 0.512 0.848 no correct answerThe Ksp of Zn(OH)2 is 3.00 x 10^-173. Calculate the difference between ΔH and ΔU when 1.0 mol of Sn(s, grey) of density 5.75 g cm-3 changes to Sn(s, white) of density 7.31 g cm-3 at 10.0 bar.
- What is ΔSsys for a condensation phase transition at 26.5 °C for a compound that condenses at 26.5 °C and the ΔHsys = -40.1 kJ mol-1 for this process? Express your answer in J mol-1 K-1 to at least two significant figures.A 0.064 kg of octane vapor (MW = 114) is mixed with 0.91 kg of air ( MW = 29 ) in the manifold is 86.1 kPa, and the temperature is 290 K. Assume octane behaves ideally, what is the total volume of this mixture. Show unit analysis and conversion on solution. Round off to four decimal places.. If the enthalpy change of an enzymatic reaction associated with the conversion of 2000 g of a target analyte substrate to 1 mol of the product is -100 kj/mol, the heat capacity of the system is 1 kj/K.Kg, Seebeck coefficient is 0.01 V/K, then estimate the output potential difference (ΔV) of a thermopile, with one pair number of the thermocouple.
- What is the retardation factor of a pulse of dissolved benzene move through organic-rich wetland soil (organic carbon fraction of soil, foc=50%) if the effective porosity is 0.4 and the bulk density of the soil is 1.5 g/cm3. Follow these important hints: - Determine organic carbon partition coefficient (Koc) - Use this Equation: K oc = K d / f oc to calculate Kd. Select the range which includes your calculated answer. Group of answer choices 300 to 400 2.0 to 2.5 mg/l 2.0 to 2.5 2.6 to 4.8 0.5 to 1.0 2.6 to 4.8 mg/l None of these ranges include my answer.(a) What is the significance of the triple point in a phasediagram? (b) Could you measure the triple point of waterby measuring the temperature in a vessel in which watervapor, liquid water, and ice are in equilibrium under 1 atmof air? Explain.The following concentrations of the vapour at two consecutive trays in the rectifying section of a column have been measured: y3 = 0.94 and y4 = 0.925. The equation of the operating line is y = 0.6667x + 0.32. The relative volatility at the liquid composition on the third tray is α = 1.85. Calculate the Murphree efficiency of the tray.

