3. Fill out the missing boxes in the table below, after referring to the object in Photo X (press on the hyperlink or check the attached photo: Photo X) - Solid Object geometry What object geometry do you see?) Object dimensions Object Volume (look up the formula for calculating the volume) Object Mass Calculated Density of the Object how your calculations below: Diameter = 8.8 cm Height = 25.4 cm 680.0561 g
3. Fill out the missing boxes in the table below, after referring to the object in Photo X (press on the hyperlink or check the attached photo: Photo X) - Solid Object geometry What object geometry do you see?) Object dimensions Object Volume (look up the formula for calculating the volume) Object Mass Calculated Density of the Object how your calculations below: Diameter = 8.8 cm Height = 25.4 cm 680.0561 g
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter1: Chemistry: An Introduction
Section: Chapter Questions
Problem 4QAP: The Chemistry in Focus segment titled Dr. Ruth—cotton Hero discusses the enormous contribution of...
Related questions
Question
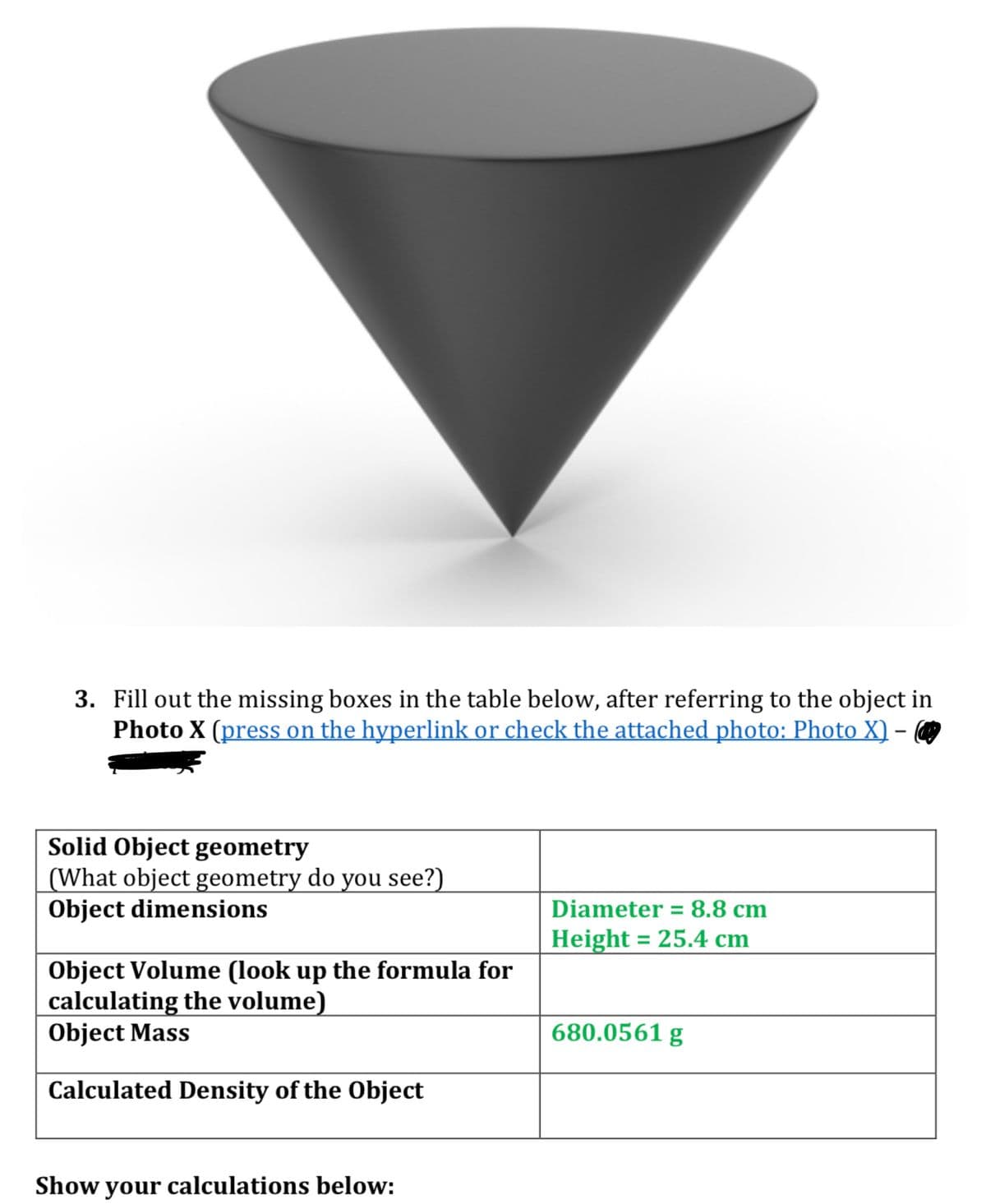
Transcribed Image Text:3. Fill out the missing boxes in the table below, after referring to the object in
Photo X (press on the hyperlink or check the attached photo: Photo X) -
Solid Object geometry
(What object geometry do you see?)
Object dimensions
Object Volume (look up the formula for
calculating the volume)
Object Mass
Calculated Density of the Object
Show your calculations below:
Diameter = 8.8 cm
Height = 25.4 cm
680.0561 g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning