For a given reaction, how will the rate constant (k) change with temperature? The rate constant will be the same at all temperatures. The rate constant will increase as temperature increases. The rate constant will decrease as temperature increases. How the rate constant changes will depend on what the specific reaction is.
For a given reaction, how will the rate constant (k) change with temperature? The rate constant will be the same at all temperatures. The rate constant will increase as temperature increases. The rate constant will decrease as temperature increases. How the rate constant changes will depend on what the specific reaction is.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 66QRT
Related questions
Question
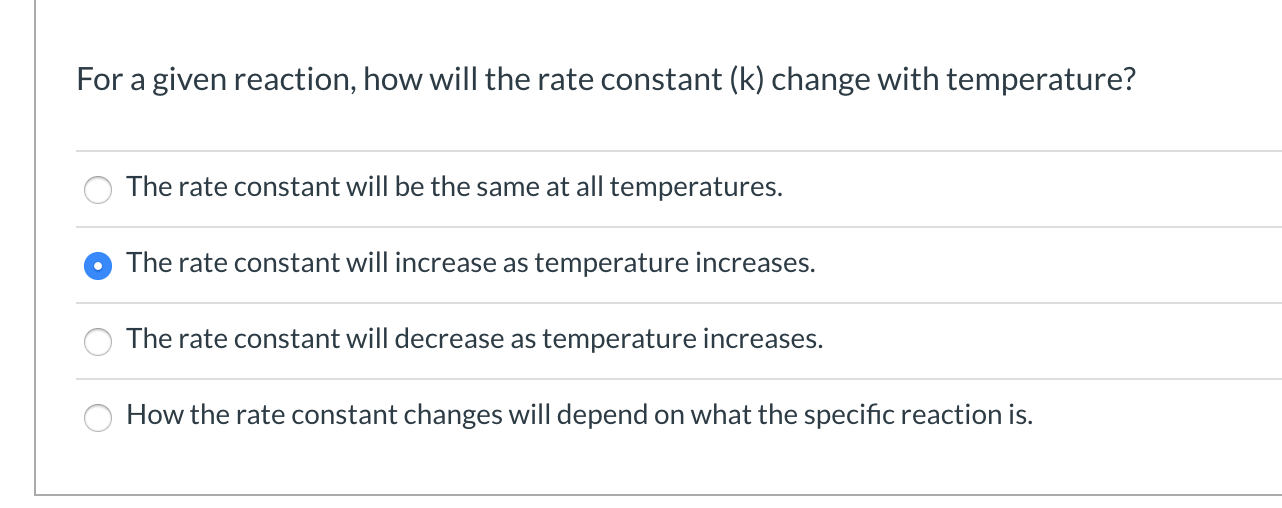
Transcribed Image Text:For a given reaction, how will the rate constant (k) change with temperature?
The rate constant will be the same at all temperatures.
The rate constant will increase as temperature increases.
The rate constant will decrease as temperature increases.
How the rate constant changes will depend on what the specific reaction is.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning