What is the rate constant for this reaction? b) If the volume of the reaction container is decreased by a factor of 2 (the volume is cut in half), will the reaction rate be faster or slower? By what factor will the rate of the reaction change?
a) What is the rate constant for this reaction?
b) If the volume of the reaction container is decreased by a factor of 2 (the volume is cut in half), will the

As, in experiment 1 and 2,
Concentration of BF3 is constant and when ammonia concentration is halved then initial rate is also halved means
Rate = k [NH3]
In experiment 4 and 5, keeping ammonia concentration constant then,
Concentration of BF3 is doubled in experiment 4 from 5, similarly rate gets doubles in experiment 4 than 5
Hence, rate = k [BF3]
This reaction is first order reaction with both reactant ammonia and BF3
Final rate = k [NH3][BF3]
Rate constant = k
- Rate = k [NH3][BF3] …(1)
Hence, substituting the values in equation 1.
Rate = 0.2130, [NH3] = 0.25 M, [BF3] = 0.25 M
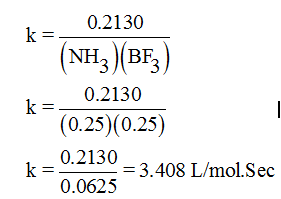
Step by step
Solved in 3 steps with 1 images









