For each example, specify whether the two structures are resonanc contributors to the same resonance hybrid. а) H3C-0-CH2 H3C-O=CH2 b) H2C=CH-CH-CH3 H2C-CH=CH-CH3
For each example, specify whether the two structures are resonanc contributors to the same resonance hybrid. а) H3C-0-CH2 H3C-O=CH2 b) H2C=CH-CH-CH3 H2C-CH=CH-CH3
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter17: Conjugation And Molecular Orbital (mo) Theory
Section: Chapter Questions
Problem 10E
Related questions
Question
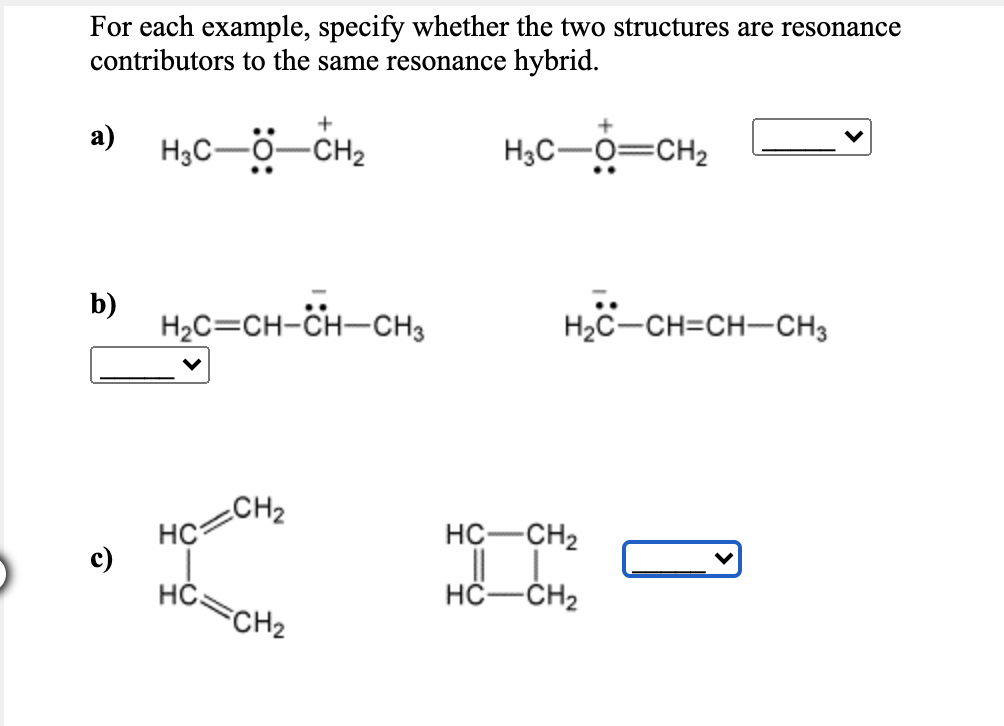
Transcribed Image Text:For each example, specify whether the two structures are resonance
contributors to the same resonance hybrid.
а)
H3C-0-CH2
H3C-O=CH2
b)
H2C=CH-CH-CH3
H2C-CH=CH-CH3
CH2
HC
c)
HČ.
FCH2
I I-
HC-CH2
HC-ČH2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning