For each of the molecules shown below, draw the acid dissociation reaction. If a conjugate base has resonance structures, draw all valid resonance structures. A A LH H* + B ii - H* + CF3 H. H* + H. Identify the stronger acid in each of the pairs below; provide an expla for each pair. A & B B & C A& C
For each of the molecules shown below, draw the acid dissociation reaction. If a conjugate base has resonance structures, draw all valid resonance structures. A A LH H* + B ii - H* + CF3 H. H* + H. Identify the stronger acid in each of the pairs below; provide an expla for each pair. A & B B & C A& C
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 13E: For each molecule below, draw the conjugate acid or conjugate base or both if the molecule hasboth a...
Related questions
Question
100%
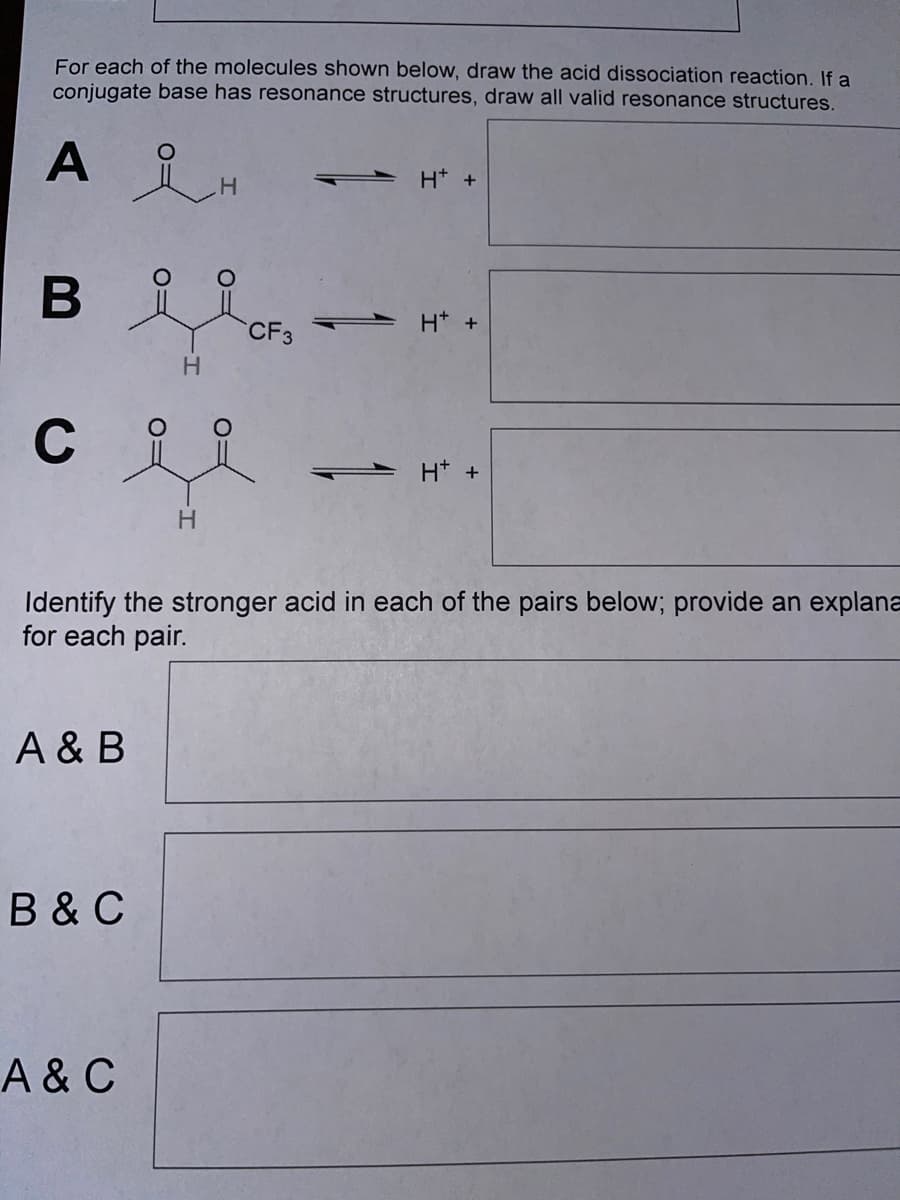
Transcribed Image Text:For each of the molecules shown below, draw the acid dissociation reaction. If a
conjugate base has resonance structures, draw all valid resonance structures.
A
H* +
H.
- H* +
CF3
H.
C
H* +
Identify the stronger acid in each of the pairs below; provide an explana
for each pair.
A & B
B &C
A& C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning