For each of the processes, determine whether the entropy of the system is increasing or decreasing. The system is underlined. Entropy is increasing Entropy is decreasing Answer Bank a snowman melts on a spring day a water bottle cools down in a refrigerator iron rusts to iron oxide a document goes through a paper shredder dissolved sugar precipitates out of water to form rock candy
For each of the processes, determine whether the entropy of the system is increasing or decreasing. The system is underlined. Entropy is increasing Entropy is decreasing Answer Bank a snowman melts on a spring day a water bottle cools down in a refrigerator iron rusts to iron oxide a document goes through a paper shredder dissolved sugar precipitates out of water to form rock candy
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter13: Spontaneous Processes And Thermodynamic Equilibrium
Section: Chapter Questions
Problem 9P
Related questions
Question
100%
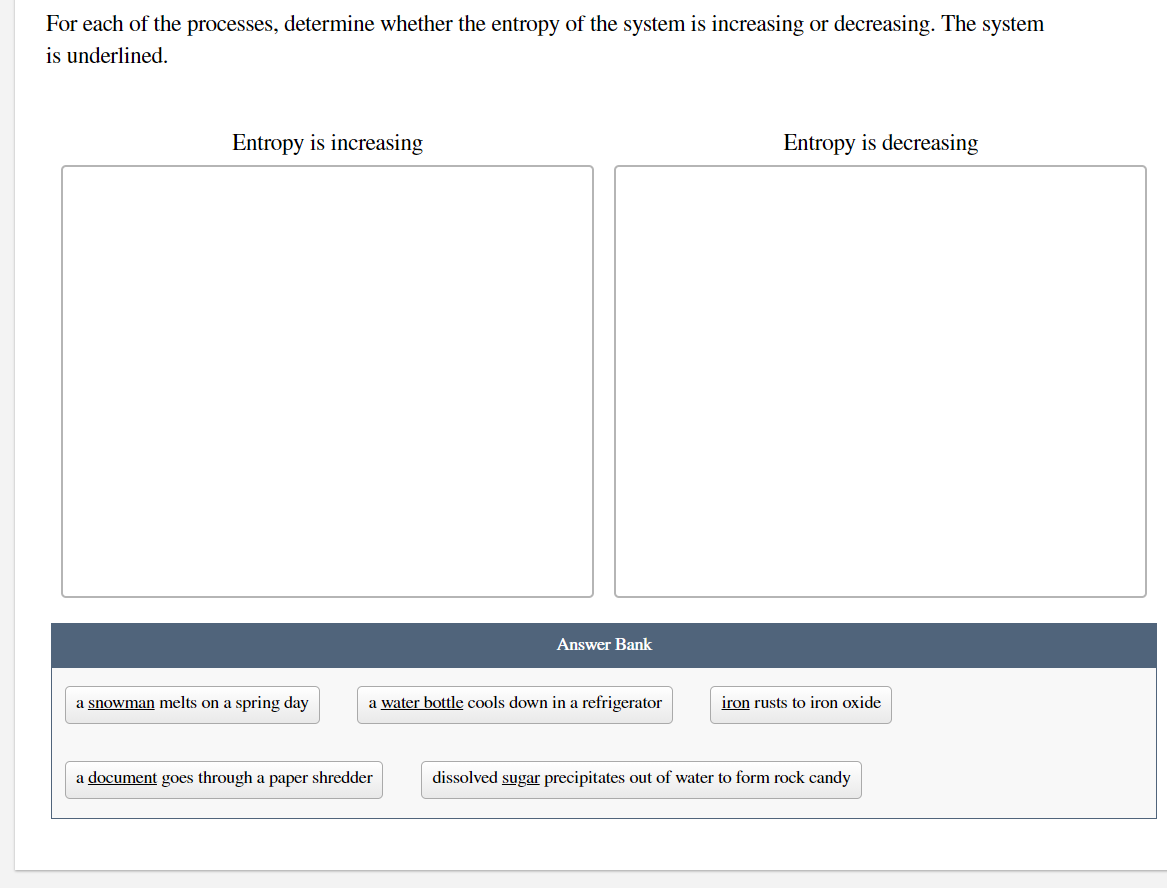
Transcribed Image Text:For each of the processes, determine whether the entropy of the system is increasing or decreasing. The system
is underlined.
Entropy is increasing
Entropy is decreasing
Answer Bank
a snowman melts on a spring day
a water bottle cools down in a refrigerator
iron rusts to iron oxide
a document goes through a paper shredder
dissolved sugar precipitates out of water to form rock candy
Expert Solution
Step 1
Here are some points to easily identify the direction of change in entropy of physical processes-
- Entropy is always assisted by the term "randomness" and "disorder". So, an increase in these two in any case will always be assisted with an increase in the entropy of the system,
- So, from the above point, we can figure it out easily that, solid substances are more ordered and with least entropy, then comes liquid and at last the gases are the most disordered. So, on going from, solid>liquid then gas, entropy always increases,
- Heating is always associated with an increase in entropy and cooling is associated with a decrease in entropy,
- In general, spontaneous processes are associated with an increase in entropy of the system(though not always!) and the processes that involve any external force i.e. non-spontaneous processes are associated with decrease in entropy.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning